
(a)
Interpretation: The number of triple points in the given phase diagram of carbon needs to be determined.
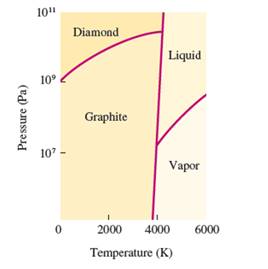
Concept Introduction:
The conversion of
The heating -cooling curve is the curve of temperature v/s time that interpret the change in the states of matter with increase in temperature.
Freezing point is the temperature at which the solid state converts to liquid state or vice-versa. Boiling point is the temperature at which the liquid and gas state reach to an equilibrium hence after this temperature, both states must be in equilibrium.
(b)
Interpretation: The phases which coexist at the triple points in the given phase diagram of carbon needs to be determined.
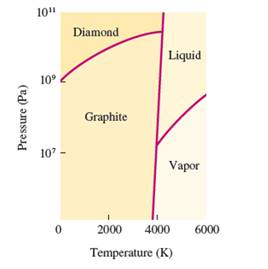
Concept Introduction:
The conversion of solid to liquid and gas involves the change in temperature that can display in heat curve.
The heating -cooling curve is the curve of temperature v/s time that interpret the change in the states of matter with increase in temperature.
Freezing point is the temperature at which the solid state converts to liquid state or vice-versa. Boiling point is the temperature at which the liquid and gas state reach to an equilibrium hence after this temperature, both states must be in equilibrium.
(c)
Interpretation: The change when graphite is subjected at very high pressure at room temperature needs to be explained.
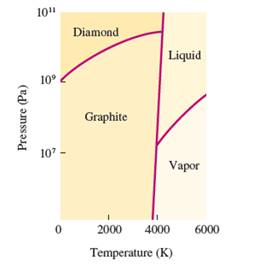
Concept Introduction:
The conversion of solid to liquid and gas involves the change in temperature that can display in heat curve.
The heating -cooling curve is the curve of temperature v/s time that interpret the change in the states of matter with increase in temperature.
Freezing point is the temperature at which the solid state converts to liquid state or vice-versa. Boiling point is the temperature at which the liquid and gas state reach to an equilibrium hence after this temperature, both states must be in equilibrium.
(d)
Interpretation: The denser state out of graphite and diamond needs to be determined, if the density increases with increasing the pressure.
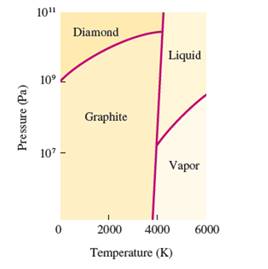
Concept Introduction:
The conversion of solid to liquid and gas involves the change in temperature that can display in heat curve.
The heating -cooling curve is the curve of temperature v/s time that interpret the change in the states of matter with increase in temperature.
Freezing point is the temperature at which the solid state converts to liquid state or vice-versa. Boiling point is the temperature at which the liquid and gas state reach to an equilibrium hence after this temperature, both states must be in equilibrium.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Chemical Principles
- pls help asaparrow_forwardpls help asaparrow_forward9. Consider the following galvanic cell: Fe (s) | Fe(NO3)2 (aq) || Sn(NO3)2 (aq) | Sn (s) a. Write an equation for the half reactions occurring at the anode and cathode. b. Calculate the standard cell potential Show all of your work. c. Draw and label the galvanic cell, including the anode and cathode, direction of electron flow, and direction of ion migration.arrow_forward
- pls help asaparrow_forward11. Use the equation below to answer the following questions: 2 Al(s) + 3 Cd(NO3)2 (aq) → 2 Al(NO3)3 (aq) + 3 Cd(s) a. What is the net ionic equation for the reaction? b. Which species is a spectator ion in this reaction? Define a spectator ion. c. Identify the oxidizing agent and the reducing agent.arrow_forwardpls help asaparrow_forward
- 22arrow_forwardPLEASE READ!!! I DONT WANT EXAMPLES, I DONT WANT WORDS OR PARAGRAPHS FOR THE MECHANISM!!! THANKS First image: QUESTION 6. I have to show, with ARROWS and STRUCTURES, the mechanism of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary. I also tried to draw the mechanism, tell me what to change. Please note that its an AMIDE thats formed not an AMINE the nitrogen has ONE hydrogen and one Phenyl-C-Phenyl. I already asked for this mechanism and got as a final product ...-NH2 not whats shown on the picture, thank you Ths second part. QUESTION 3. I just need a way to synthesize the lactone A, I already started please continue from where I left it Second image: I simply need the products, substrates or reagents, thank youarrow_forwardIndicate how to prepare a 10% sodium hydroxide (NaOH) solution to a slightly alkaline pH.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





