
(a)
Interpretation:
Condensed structural formula of the organic product that is formed when pentanal is treated with hydrogen in presence of nickel catalyst has to be given.
Concept Introduction:
In
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
The reverse of
(b)
Interpretation:
Condensed structural formula of the organic product that is formed when pentanal is treated with ethanol in 1 to 1 reacting ratio has to be given.
Concept Introduction:
Aldehydes contain a carbonyl group that is bonded to a hydrogen atom and a carbon atom. Ketones are compounds that contain a carbonyl group bonded to two carbon atoms. Aldehydes and ketones undergo addition reaction across the carbonyl group.
Aldehydes and ketones react with alcohol to form hemiacetal as the product. This reacts with further molecule of aldehyde or ketone to form acetal.
Hemiacetal is an addition product that is obtained by reaction between aldehyde or ketone with alcohol. The general reaction of hemiacetal formation can be given as,
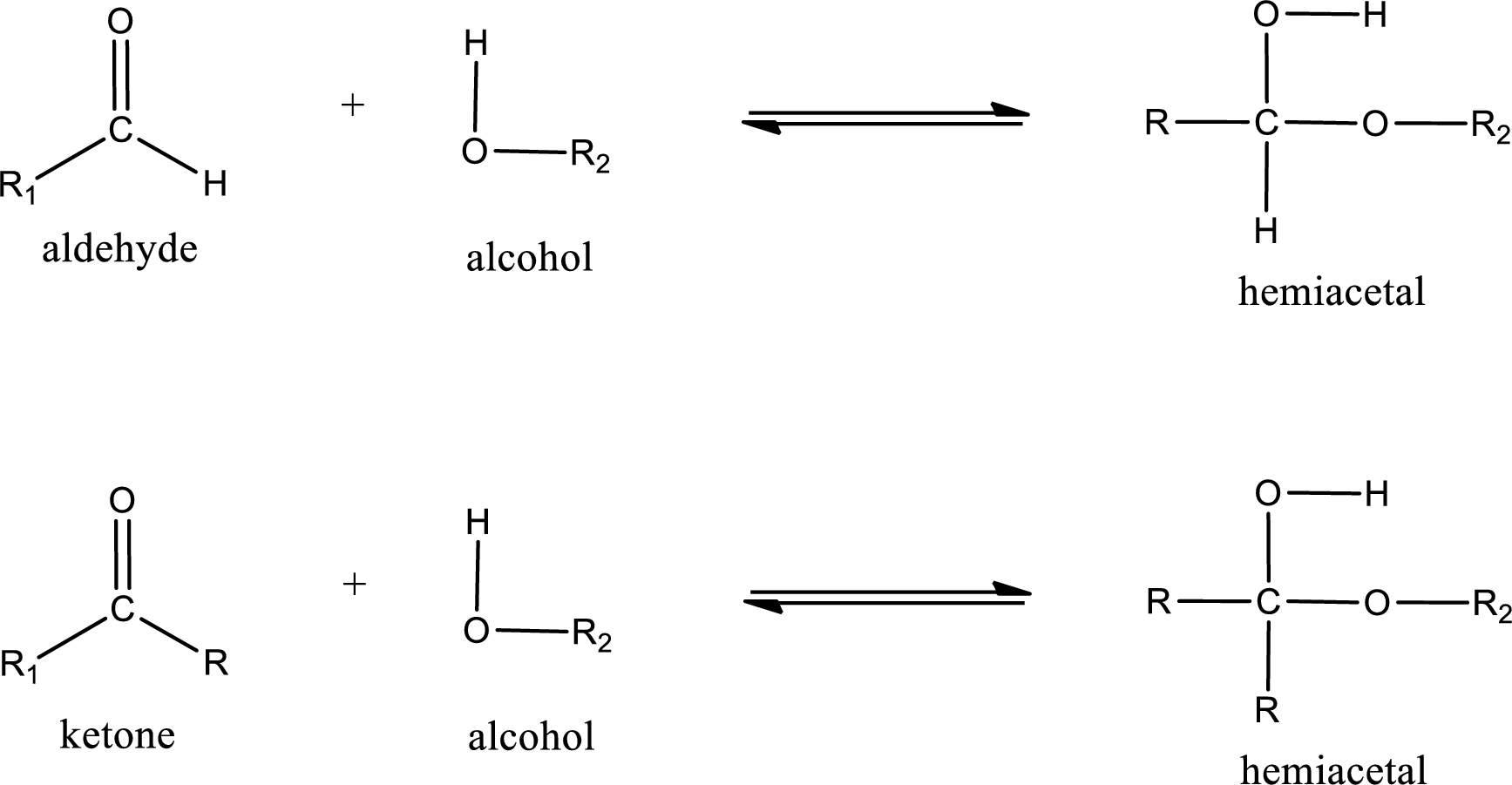
(c)
Interpretation:
Condensed structural formula of the organic product that is formed when pentanal is treated with methanol in 1 to 2 reacting ratio has to be given.
Concept Introduction:
Aldehydes contain a carbonyl group that is bonded to a hydrogen atom and a carbon atom. Ketones are compounds that contain a carbonyl group bonded to two carbon atoms. Aldehydes and ketones undergo addition reaction across the carbonyl group.
Aldehydes and ketones react with alcohol to form hemiacetal as the product. This reacts with further molecule of aldehyde or ketone to form acetal.
Hemiacetal is an addition product that is obtained by reaction between aldehyde or ketone with alcohol. The general reaction of hemiacetal formation can be given as,
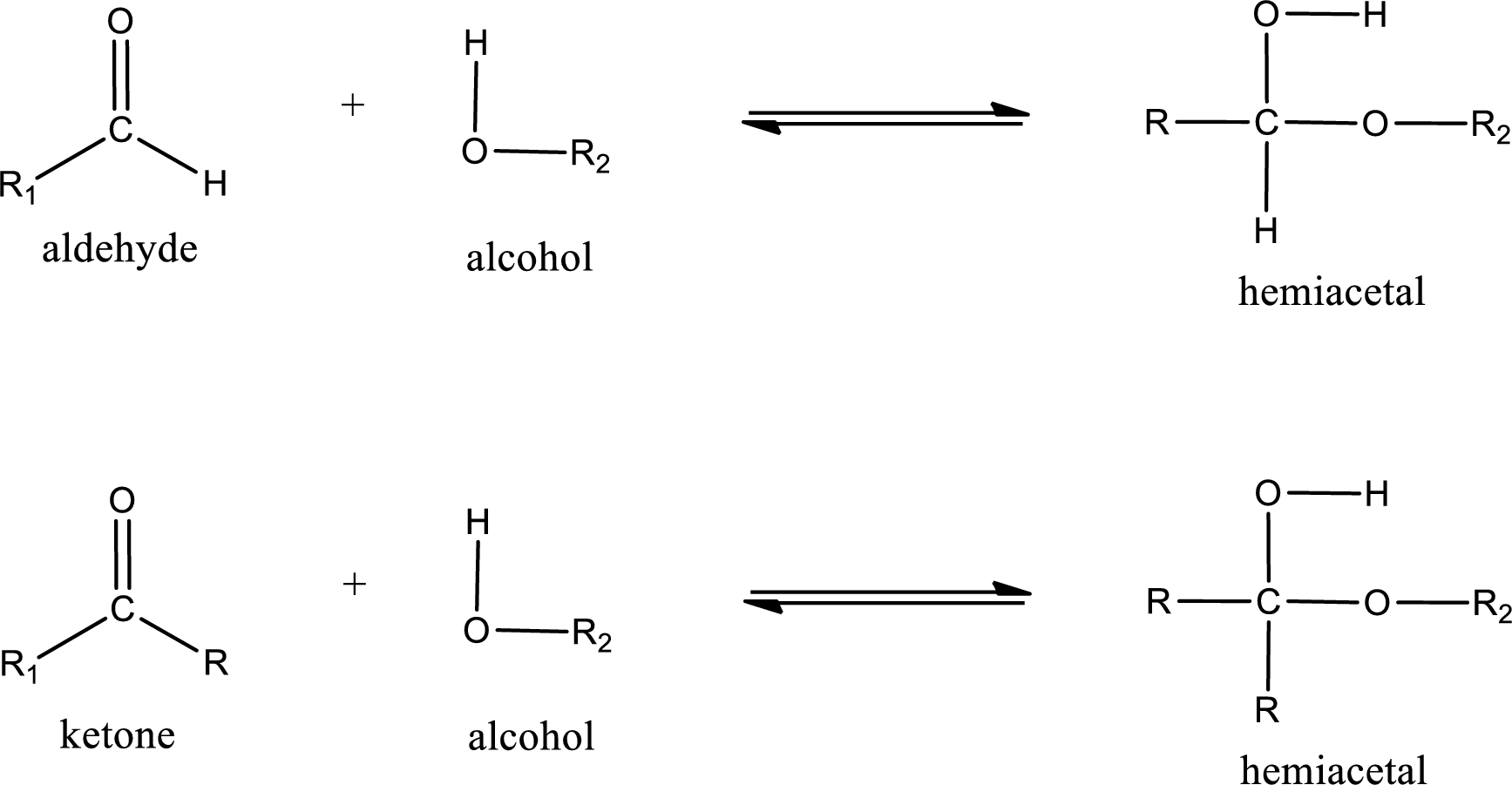
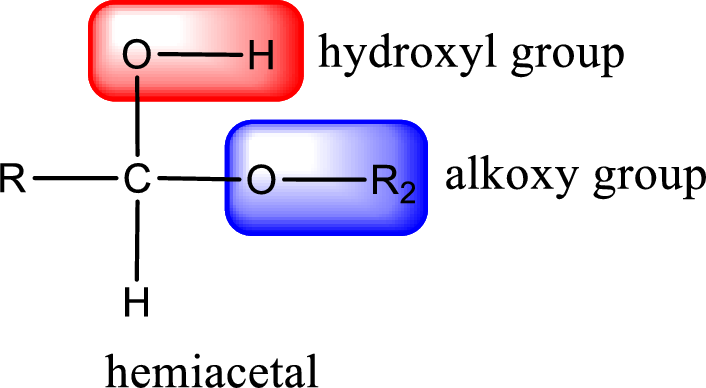
From the above general structure of hemiacetal it is found that it is an organic compound that contains a carbon atom that is bonded to an alkoxy group and a hydroxyl group.
Acetal is formed when the formed hemiacetal reacts with further alcohol molecule so that the hydroxyl group in the hemiacetal is converted into alkoxy group. This can be shown as given below,
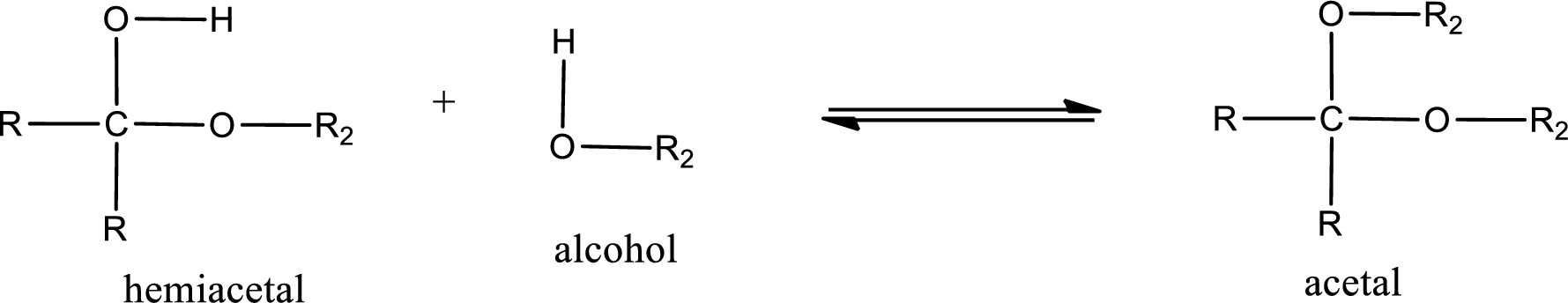
The alcohol that is reacting with the hemiacetal can be from the same alcohol which involved in formation of hemiacetal or a different alcohol molecule. Therefore, the ketone or aldehyde must react with two alcohol molecules that may or may not be identical to form acetal.
(d)
Interpretation:
Condensed structural formula of the organic product that is formed when pentanal is treated with
Concept Introduction:
In organic chemistry, oxidation reaction is referred to the number
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give aldehyde and carboxylic acid as product. Secondary alcohol undergoes oxidation to give ketone as the product.
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Trending nowThis is a popular solution!

Chapter 15 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
- please provide the structure for this problem, thank youarrow_forwardpresented by Morallen Lig Intermine the hand product for the given mution by adding atoms, bonds, nonhonding diarion panda скуль Step 3: Comp the draw the product Step 2: Agama workup Compithe 429 ملولةarrow_forwardReaction A 0,0arrow_forward
- presented by Morillon Leaning Predict the organic product for the min кусур HSC Adithane carved arnown to come than that to the condon slchroruis in acid in in aquishri with ноюarrow_forward6.15PM Sun Mar 30 K Draw the major product of this reaction. Include any relevant stereochemistry. Ignore inorganic byproducts. Problem 1 of O H [PhзPCH2CH3]*C|¯ NaH Drawing > Q Atoms, Bonds and Draw or tap a nearrow_forward8:17 PM Sun Mar 30 Draw the major product of this reaction. Ignore inorganic byproducts. HSCH2CH2CH2SH, BF3 Probler Drawing Ato Bonds Clarrow_forward
- Name the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forwardMacmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





