
(a)
Interpretation:
Molecular formula for propanal has to be given.
Concept Introduction:
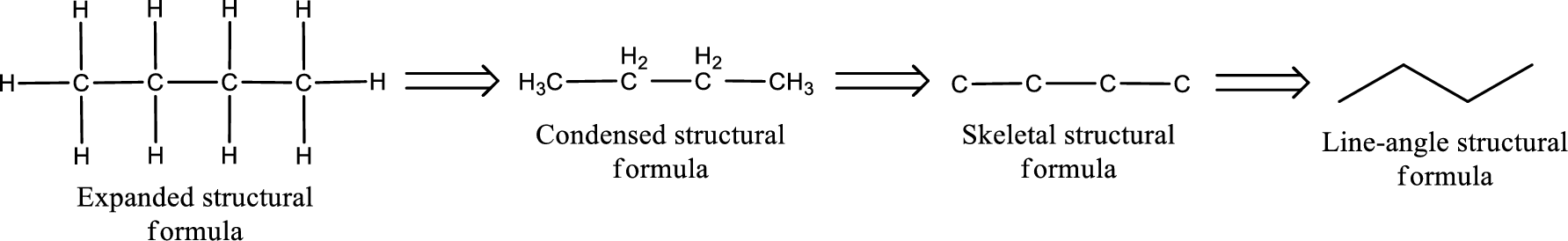
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
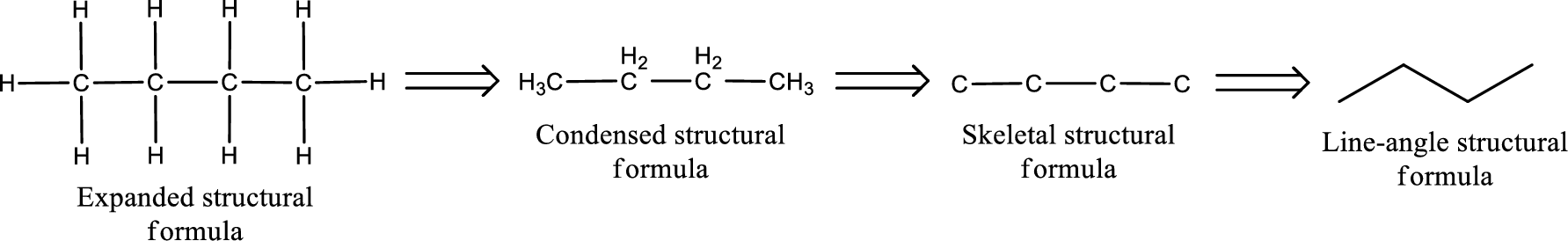
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
Molecular formula is the simple representation of different kind of atoms that are present in the compound. This is represented as the number of atoms of same kind given in the subscript followed by the other element symbol.
(a)
Answer to Problem 15.48EP
Molecular formula of propanal is
Explanation of Solution
The given name of the compound is propanal. From the name it is understood that the parent carbon chain is propane and it contains four carbon atoms. The parent chain can be drawn as shown below,
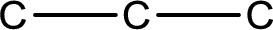
From the name of the given compound, it is identified to be an
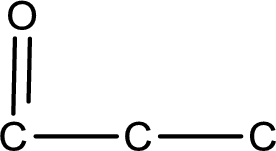
Carbon atom has a valence of four. Hence, carbon atom can form four covalent bonds. The remaining bonds are satisfied by hydrogen atom. The structure is obtained as shown below,
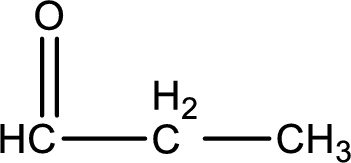
Molecular formula is found by counting the number of atoms of the same kind present in the above structure. This gives the molecular formula as
Molecular formula of propanal is given.
(b)
Interpretation:
Molecular formula for propanedial has to be given.
Concept Introduction:
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
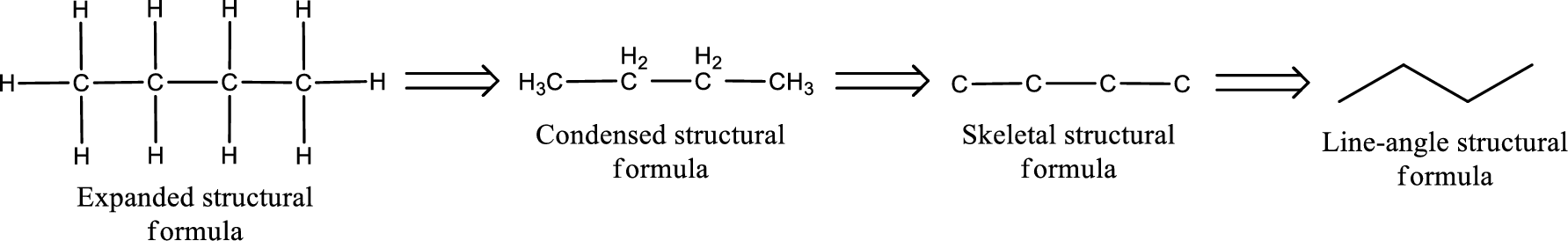
Molecular formula is the simple representation of different kind of atoms that are present in the compound. This is represented as the number of atoms of same kind given in the subscript followed by the other element symbol.
(b)
Answer to Problem 15.48EP
Molecular formula of propanedial is
Explanation of Solution
The given name of the compound is propanedial. From the name it is understood that the parent carbon chain is propane and it contains three carbon atoms. The parent chain can be drawn as shown below,
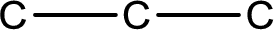
From the name of the given compound, it is identified to be an aldehyde. The carbonyl carbon atom is the first carbon atom and third carbon atom.
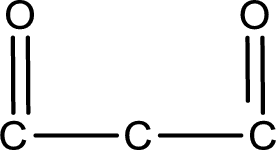
Carbon atom has a valence of four. Hence, carbon atom can form four covalent bonds. The remaining bonds are satisfied by hydrogen atom. The structure is obtained as shown below,
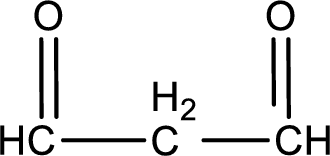
Molecular formula is found by counting the number of atoms of the same kind present in the above structure. This gives the molecular formula as
Molecular formula of propanedial is given.
(c)
Interpretation:
Molecular formula for propanone has to be given.
Concept Introduction:
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
Molecular formula is the simple representation of different kind of atoms that are present in the compound. This is represented as the number of atoms of same kind given in the subscript followed by the other element symbol.
(c)
Answer to Problem 15.48EP
Molecular formula of propanone is
Explanation of Solution
The given name of the compound is propanone. From the name it is understood that the parent carbon chain is propane and it contains three carbon atoms. The parent chain can be drawn as shown below,
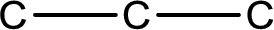
From the name of the given compound, it is identified to be a
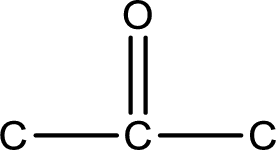
Carbon atom has a valence of four. Hence, carbon atom can form four covalent bonds. The remaining bonds are satisfied by hydrogen atom. The structure is obtained as shown below,
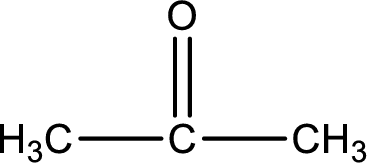
Molecular formula is found by counting the number of atoms of the same kind present in the above structure. This gives the molecular formula as
Molecular formula of propanone is given.
(d)
Interpretation:
Molecular formula for 2,4-pentanedione has to be given.
Concept Introduction:
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
Molecular formula is the simple representation of different kind of atoms that are present in the compound. This is represented as the number of atoms of same kind given in the subscript followed by the other element symbol.
(d)
Answer to Problem 15.48EP
Molecular formula of 2,4-pentanedione is
Explanation of Solution
The given name of the compound is 2,4-pentanedione. From the name it is understood that the parent carbon chain is pentane and it contains five carbon atoms. The parent chain can be drawn as shown below,

From the name of the given compound, it is identified to be a diketone with carbonyl carbon atom as second and fourth carbon atom.
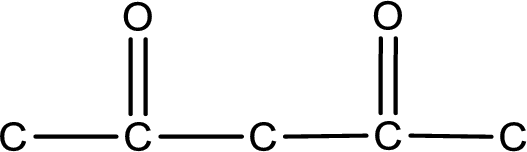
Carbon atom has a valence of four. Hence, carbon atom can form four covalent bonds. The remaining bonds are satisfied by hydrogen atom. The structure is obtained as shown below,
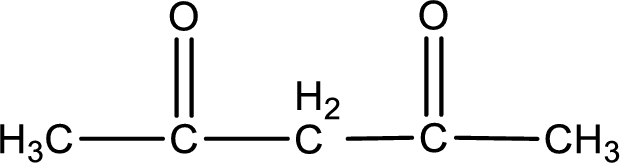
Molecular formula is found by counting the number of atoms of the same kind present in the above structure. This gives the molecular formula as
Molecular formula of 2,4-pentanedione is given.
Want to see more full solutions like this?
Chapter 15 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

