
Concept explainers
(a)
Interpretation:
Structural formula for the given
Concept Introduction:
Structure of the ketone can be drawn from the IUPAC name. In the IUPAC name, the parent chain of carbon atom can be identified and then the substituents present in it can also be identified. With these information, the structure for the given compound can be drawn. In a ketone the counting has to be done so that the carbonyl carbon atom gets the least numbering.
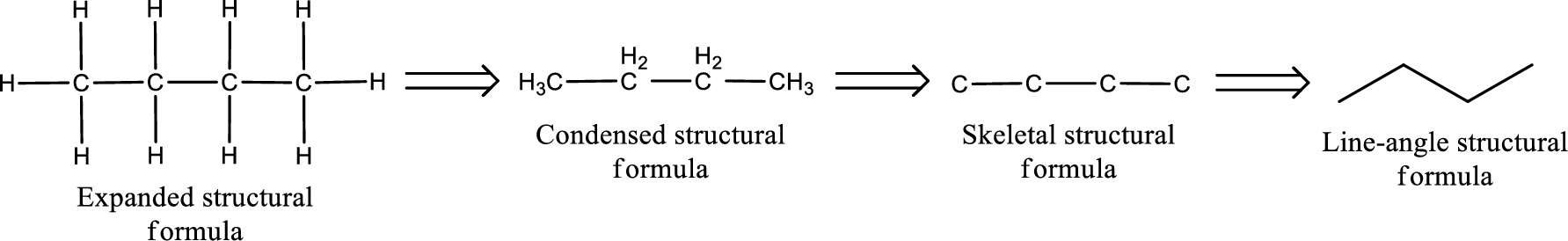
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
(a)
Answer to Problem 15.37EP
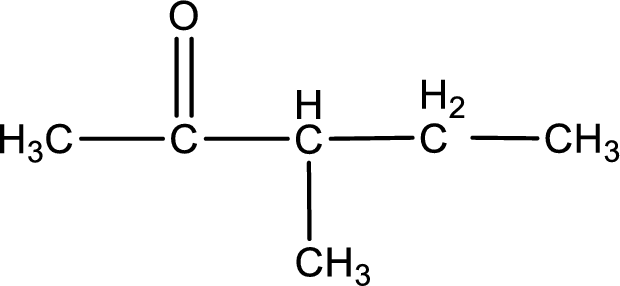
The structural formula for 3-methyl-2-pentanone is,
Explanation of Solution
The given name of the compound is 3-methyl-2-pentanone. From the name it is understood that the parent carbon chain is pentane and it contains five carbon atoms. The parent chain can be drawn as shown below,

From the name of the given ketone, the substituents that are present can be identified. In this case, the substituent is a methyl group on third carbon atom. The carbonyl carbon atom is the second carbon atom.
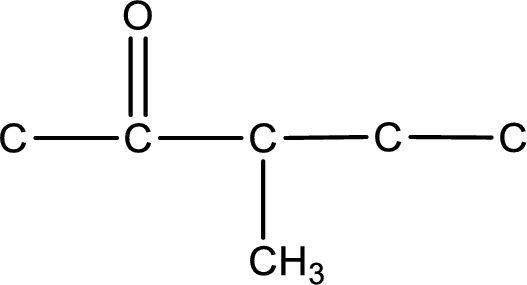
Carbon atom has a valence of four. Hence, carbon atom can form four covalent bonds. The remaining bonds are satisfied by hydrogen atom. The structure is obtained as shown below,
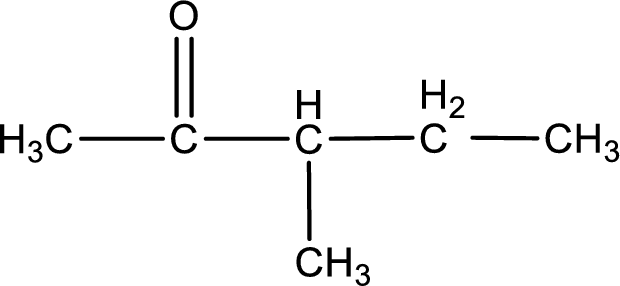
Structural formula for the given ketone is drawn.
(b)
Interpretation:
Structural formula for the given ketone has to be drawn.
Concept Introduction:
Structure of the ketone can be drawn from the IUPAC name. In the IUPAC name, the parent chain of carbon atom can be identified and then the substituents present in it can also be identified. With these information, the structure for the given compound can be drawn. In a ketone the counting has to be done so that the carbonyl carbon atom gets the least numbering.
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
(b)
Answer to Problem 15.37EP
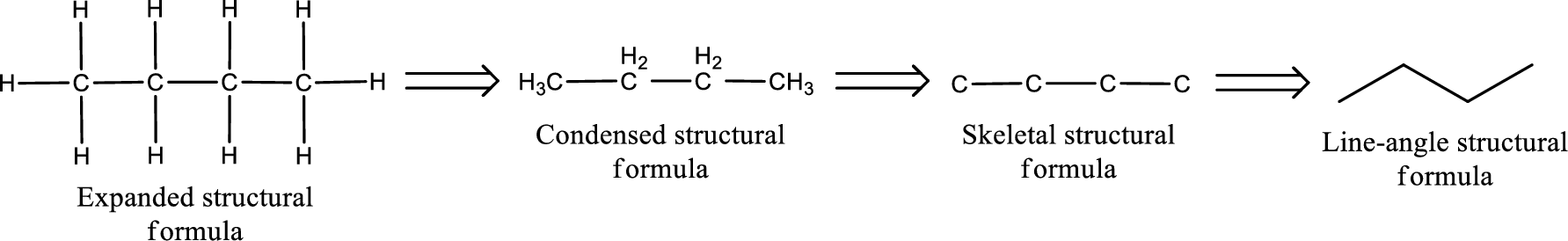
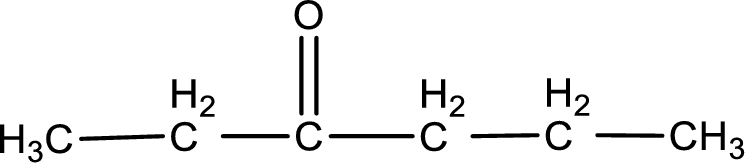
The structural formula for 3-hexanone is,
Explanation of Solution
The given name of the compound is 3-hexanone. From the name it is understood that the parent carbon chain is hexane and it contains six carbon atoms. The parent chain can be drawn as shown below,

From the name of the given ketone, the substituents that are present can be identified. In this case, there are no substituents. The carbonyl carbon atom is the third carbon atom.
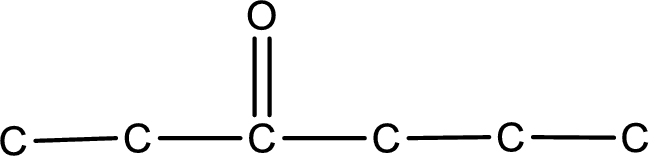
Carbon atom has a valence of four. Hence, carbon atom can form four covalent bonds. The remaining bonds are satisfied by hydrogen atom. The structure is obtained as shown below,
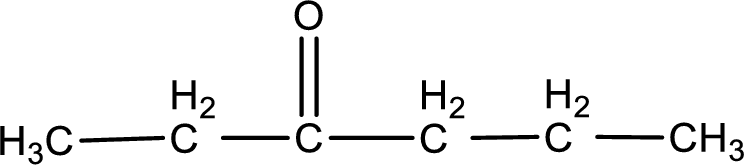
Structural formula for the given ketone is drawn.
(c)
Interpretation:
Structural formula for the given ketone has to be drawn.
Concept Introduction:
Structure of the ketone can be drawn from the IUPAC name. In the IUPAC name, the parent chain of carbon atom can be identified and then the substituents present in it can also be identified. With these information, the structure for the given compound can be drawn. In a ketone the counting has to be done so that the carbonyl carbon atom gets the least numbering.
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
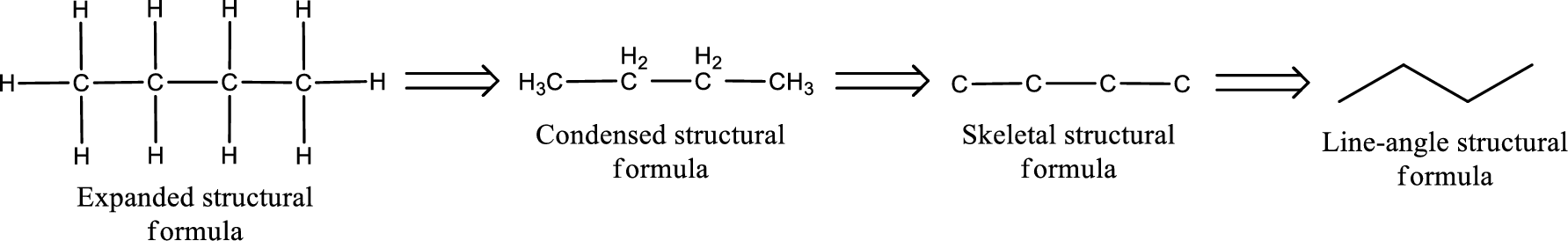
(c)
Answer to Problem 15.37EP
The structural formula for cyclobutanone is,
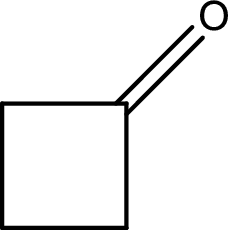
Explanation of Solution
The given name of the compound is cyclobutanone. From the name it is understood that the parent carbon chain is cyclic carbon chain that contains four carbon atoms. The parent chain can be drawn as shown below,

From the name of the given ketone, the substituents that are present can be identified. In this case, there are no substituents. The carbonyl carbon atom is any one of the carbon atom. Therefore, the structural formula of cyclobutanone can be drawn as shown below,
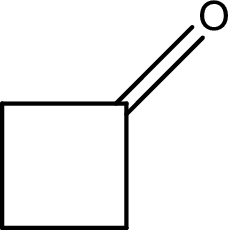
Structural formula for the given ketone is drawn.
(d)
Interpretation:
Structural formula for the given ketone has to be drawn.
Concept Introduction:
Structure of the ketone can be drawn from the IUPAC name. In the IUPAC name, the parent chain of carbon atom can be identified and then the substituents present in it can also be identified. With these information, the structure for the given compound can be drawn. In a ketone the counting has to be done so that the carbonyl carbon atom gets the least numbering.
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- • Expanded structural formula
- • Condensed structural formula
- • Skeletal structural formula
- • Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
(d)
Answer to Problem 15.37EP
The structural formula for chloropropanone is,
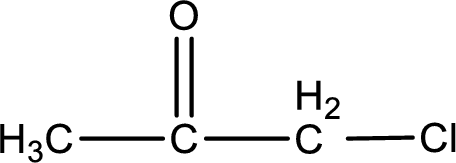
Explanation of Solution
The given name of the compound is chloropropanone. From the name it is understood that the parent carbon chain is propane and it contains three carbon atoms. The parent chain can be drawn as shown below,
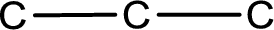
From the name of the given ketone, the substituents that are present can be identified. In this case a chlorine atom is present as a substituent. The carbonyl carbon atom is the second carbon atom as that is the only possibility.
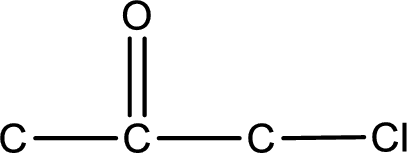
Carbon atom has a valence of four. Hence, carbon atom can form four covalent bonds. The remaining bonds are satisfied by hydrogen atom. The structure is obtained as shown below,
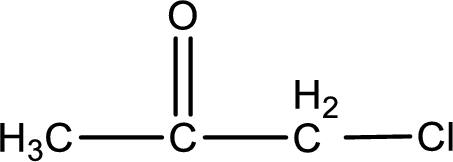
Structural formula for the given ketone is drawn.
Want to see more full solutions like this?
Chapter 15 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
- Using the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forwardPlease help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





