
Concept explainers
a.
Interpretation:
Chiral centers in the given monosaccharide has to be labelled, the monosaccharide has to be classified as D or L, the enantiomer of the given monosaccharide has to be drawn and also the Fischer projection formula has to be given.
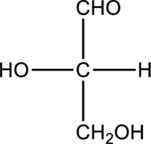
Concept Introduction:
A carbon atom that is bonded to four different groups is known as a chiral carbon atom. This can rotate the plane polarized light. D- and L- isomers of monosaccharide can be identified by looking into the chiral center that is farther from the carbonyl group. In a Fischer projection, if the
Enantiomers are two stereoisomers of a compound that rotate the plane polarized light exactly opposite. The configuration present in the enantiomers will be exactly opposite to each other.
Fischer projection formula simply uses cross for representing tetrahedral carbon atom. The carbon atom that is present in the intersection point in cross. The horizontal bonds means they are coming forward and they are present on wedge bond. The vertical bonds means they are pointing away and they are present on dashed lines.
b.
Interpretation:
Chiral centers in the given monosaccharide has to be labelled, the monosaccharide has to be classified as D or L, the enantiomer of the given monosaccharide has to be drawn and also the Fischer projection formula has to be given.
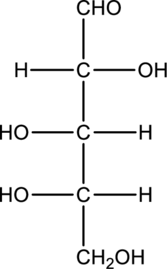
Concept Introduction:
Refer part “a.”.
c.
Interpretation:
Chiral centers in the given monosaccharide has to be labelled, the monosaccharide has to be classified as D or L, the enantiomer of the given monosaccharide has to be drawn and also the Fischer projection formula has to be given.
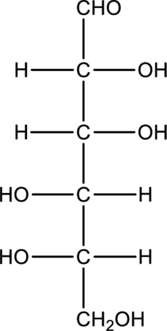
Concept Introduction:
Refer part “a.”.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Principles of General, Organic, Biological Chemistry
- Indicate the products obtained by reacting ethylbenzene with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained when tert-butylbenzene reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained when acetophenone reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forward
- Indicate the products obtained from the reaction of N-(4-methylphenyl)acetamide with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forwardIndicate the products obtained from the reaction of 4-(trifluoromethyl)benzonitrile with a sulfonitric mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forwardIndicate the products obtained in the reaction of p-Toluidine with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forward
- Indicate the products obtained from the reaction of 4-methylbenzonitrile with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forwardIndicate the products obtained from the reaction of 2-nitrophenol with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forwardIn organic chemistry, what is the correct name for the mixture H2SO4 + HNO3 used in reactions: sulphonitric mixture or sulfonitric mixture?arrow_forward
- Formulate the products obtained by reacting p-toluidine with a sulfonate mixture. Indicate the majority if necessary.arrow_forwardConsider this organic reaction: OH Draw the major products of the reaction in the drawing area below. If there won't be any major products, because this reaction won't happen at a significant rate, check the box under the drawing area instead. Click and drag to start drawing a structure. x 0: の Carrow_forwardExplain the reasons for a compound's greater or lesser reactivity toward electrophilic aromatic substitution. Give reasons.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





