
Concept explainers
a.
Interpretation:
Compound that is formed when the given aldose is treated with hydrogen in presence of palladium as catalyst has to be drawn.
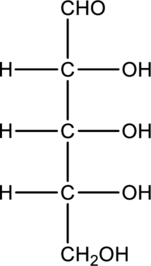
Concept Introduction:
Carbohydrates undergo reduction similar to an
b.
Interpretation:
Compound that is formed when the given aldose is treated with hydrogen in presence of palladium as catalyst has to be drawn.
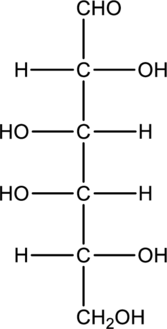
Concept Introduction:
Refer to part “a.”.
c.
Interpretation:
Compound that is formed when the given aldose is treated with hydrogen in presence of palladium as catalyst has to be drawn.
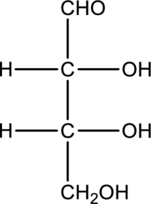
Concept Introduction:
Refer to part “a.”.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Principles of General, Organic, Biological Chemistry
- Can I please get help with this.arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


