
(a)
Interpretation:
For the given alloy, an artificial age-hardening heat treatment needs to be recommended.
Concept Introduction:
The mixture of magnesium with other elements is known as magnesium alloy. The other elements include Zinc, Manganese, Aluminum and Copper.
Solidification takes place after complete mixing. It gets phase transformation to Liquid phase and there is no diffusion in the solid phase. With a decrease in the temperature of aluminum, the solubility of aluminium in magnesium also decreases.
If temperature is increased above,
Answer to Problem 12.30P
For artificial aging, the temperatures of solvus and eutectic point is determined. For this the curve between the percentage of weight of magnesium and the temperature needs to be drawn.
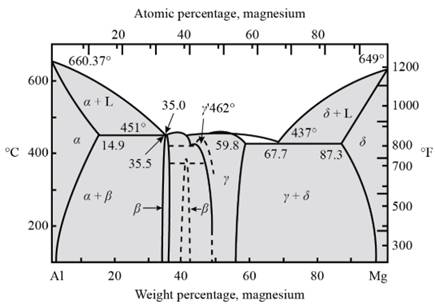
Fig. 1- Phase diagram of Al-Mg system
Explanation of Solution
Form the above graph, one gets to know all the temperatures for artificial age hardening of Magnesium-Aluminium alloy
| Temperature | Al- Mg | Al- Mg | Al- Mg |
| Treat at | Quenching | Quenching | Quenching |
| Age at | Temperature below | Temperature below | Temperature below |
Table No.
Thus, artificial age hardening depends on temperature of solid solubility and the eutectic point. Precipitation treat hardening is done by the help of quenching process.
(b)
Interpretation:
The amount of beta precipitates in all weight percentage of magnesium needs to be compared.
Concept Introduction:
The mixture of magnesium with other elements is known as magnesium alloy. The other elements include Zinc, Manganese, aluminum and Copper.
Solidification takes place after complete mixing. It gets phase transformation to Liquid phase and there is no diffusion in the solid phase. With a decrease in the temperature of aluminum, the solubility of aluminum in magnesium also decreases.
If temperature is increased above,
Answer to Problem 12.30P
The amount of beta precipitates in Al-
Explanation of Solution
Consider,
Aging temperature -
Tie line is passing through weight percentage of Mg as -
Hence, now one can calculate beta precipitate as −
For Al-
For Al-
And for Al-
From calculations, one can observe that if the weight percentage of magnesium in aluminum alloy increases then the amount of beta precipitate also increases.
(c)
Interpretation:
The requirement which is not satisfied in age hardening needs to be explained.
Concept Introduction:
The mixture of magnesium with other elements is known as magnesium alloy. The other elements include Zinc, Manganese, Aluminum and Copper.
Solidification takes place after complete mixing. It gets phase transformation to Liquid phase and there is no diffusion in the solid phase. With a decrease in the temperature of aluminum, the solubility of aluminum in magnesium also decreases.
If temperature is increased above,
Answer to Problem 12.30P
After the heat treatment, it can be observed that there is a little strengthening remains because the coherent precipitation is not formed.
Explanation of Solution
Age hardening is used to increase the yield strength of malleable metals like as −
- Aluminium
- Magnesium
- Nickel
- Titanium
The hardening depends on the temperature and its solubility in solid phase which results into pure or fine particles with the removal of the impurity phase. For precipitation, sometimes the alloy is kept at the elevated temperature for hours. This process of time delay is known as Aging.
After testing of the alloys with heat treatment, some amount of strengthening is observed. At this stage there is no formation of coherent precipitation. There is only simple dispersion strength as compared to the age hardening.
Want to see more full solutions like this?
Chapter 12 Solutions
Essentials of Materials Science and Engineering, SI Edition
- boston csv is located here: https://gist.github.com/nnbphuong/def91b5553736764e8e08f6255390f37cross-validation lab Remember that the best model has the highest accuracy (mean) and the lowest variance among all models to avoid overfitting. ''' # Compare Algorithms from pandas import read_csv from matplotlib import pyplot from sklearn.model_selection import KFold from sklearn.model_selection import cross_val_score from sklearn.ensemble import RandomForestClassifier ############################################################################ # to do: add the linear regression model (import the linear regression model from sklearn) ############################################################################# # load dataset. You should put the dataset in the current directory or provide a path to the where # the data is located. filename = 'Diabetes dataset.csv' names = ['preg', 'plas', 'pres', 'skin', 'test', 'mass', 'pedi', 'age', 'class'] dataframe = read_csv(filename, names=names)…arrow_forwardhelp me with line search method in A.3arrow_forwardNeed help with the cross-validation lab in the attachment. boston housing is located here: https://gist.github.com/nnbphuong/def91b5553736764e8e08f6255390f37arrow_forward
- 1. A W10x60 with sections properties shown is to be used as a column. If the unsupported length is 5.0m, find the Safe Axial Load that can be carried by the section. Use Fy=248 MPa and K=1.50 (35pts) Section Properties: A = 11355 mm2 Ix = 142x106 mm4 rx = 111.51 mm Iy = 48 x 106 mm4 ry = 65.28 mm 2. A Steel Column will be required to carry a total load of 500KN. If a tubular section will be utilized and the required dimension must not exceed 400x400, what will be the required thickness of the section (tf=tw). Use Fy=248 MPa L=4.5m, K=1.0 and 80% of initial fa will be used. (50pts) Note: Plate thickness available are: 2mm, 3mm, 4mm, 6mm, 8mm and 10mm 3. A steel tension rod will be subjected to a tension load of 320 KN. What will be the required diameter of the rod if Fy=248 MPa (15pts)arrow_forwardExample A continuous fractionating column is to be design to separate 30 Ib/h of a mixture of 40 percent benzene and 60 percent toluene into an overhead product containing 97 percent benzene and a bottom product containing 98 percent toluene. These percentages are by weight. A reflux ratio of 3.5 mol to 1 mol of product is to be used. The molal latent heats of benzene and toluene are 7,360 and 7,960 cal/mol, respectively. Benzene and toluene form an ideal system with a relative volatility of about 2.5; the equilibrium curve is shown in Figure below. The feed has a boiling point of 95 °C at a pressure of 1 atm. (a) Calculate the moles of overhead product and bottom product per hour. (b) Determine the number of ideal plate and the position of the feed plate: (i) if the feed is liquid and at its boiling point. (ii)if the feed is liquid and at 20 °C (specific heat 0.44 cal/ g.°C) (iii) if the feed is a mixture of two-thirds vapor and one-third liquid. (c) If steam at 20 Ib/in? (1.36 atm)…arrow_forwardfor purposes of orientation every contour may must display what?arrow_forward
- This is what I'm carrying out my project in, what would be some good sources to use as credible case studies, white papers and research papers. The Role of Artificial Intelligence in Engineering: A Focus on Manufacturing, Automation, and the Automotive Industry within the Context of Industry 4.0arrow_forwardthis is an old practice exam, the answer is Ax = -4, Ay = -12,Az = 32.5, Bx= 34, Bz = 5, By = 0 but how?arrow_forwardThis is an old practice exam, the answer is Ax = Az = 0, Ay = 2000, TDE = 4750, Cx = 2000, Cy = 2000, Cz = -800 but how?arrow_forward
- During calibration of an LVDT, the data shown in the accompanying table were obtained. Using a spreadsheetprogram, plot the relation between the micrometer reading and voltage. What is the linear range of the LVDT? Determinethe calibration factor of the LVDT by obtaining the best fit line of the data within the linear range.arrow_forwardHydrogen, important for numerous processes, can be produced by the shift reaction: CO+H2O-CO2 +H2 In the reactor system shown in the Figure, the conditions of conversion have been adjusted so that the H2 content of the effluent from the reactor is 3 mol %. Based on the data in following figure: a. Calculate the composition of the fresh feed. b. Calculate the moles of recycle per mole of hydrogen produced. Recycle CO, H2O Feed CO H₂O Reactor % 3 mol % H₂ Separator % CO₂ 48 H₂ 48 CO 4arrow_forwardQ2. Figure Q2 shows a block diagram with an input of C(s) and an output R(s). a) C(s) K₁ R(s) K2 1 + 5s 1+2s Figure Q2. Block diagram of control system. Simply the block diagram to get the transfer function of the system C(s)/R(s). b) What is the order of the system? c) What is the gain of the system? d) Determine the values of K₁ and K₂ to obtain a natural frequency w of 0.5 rad/s and damping ratio of 0.4. e) What is the rise time and overshoot of the system with a unit step input?arrow_forward
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





