
(a)
Interpretation:
The amount, composition and hardness of material at
Concept Introduction:
Material hardness is the property of a metal due to which material resist the plastic deformation. Plastic deformation means material deformation which undergoes non-reversible change. Hardness is the property of any material which process stiffness resistance to bending, scratching or cutting. Hardness is not constant or fixed for all material, but it depends upon strength and plasticity of metal. Material hardness is expressed in terms of hardness number.
Answer to Problem 12.102P
The composition, hardness and amount are
Explanation of Solution
Given:
Graph showing
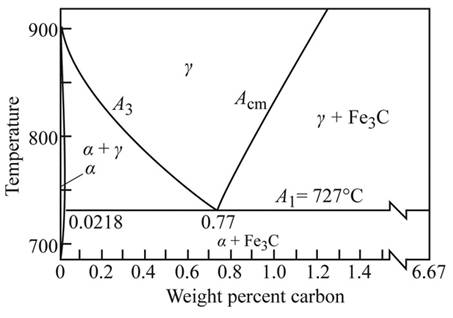
As shown in the given diagram, at the line
As from the figure, the effect of hardness of martensite in steel, the hardness of martensite at
Where,
Put, the values in lever rule,
(b)
Interpretation:
The amount, composition and hardness of material at
Concept Introduction:
Material hardness is the property of a metal due to which material resist the plastic deformation. Plastic deformation means material deformation which undergoes non-reversible change. Hardness is the property of any material which process stiffness resistance to bending, scratching or cutting. Hardness is not constant or fixed for all material, but it depends upon strength and plasticity of metal. Material hardness is expressed in terms of hardness number.
Answer to Problem 12.102P
The composition, hardness and amount are
Explanation of Solution
From the given figure, draw a line at
The lever rule is given by,
Where,
Put the values in lever rule
(c)
Interpretation:
The amount, composition and hardness of material at
Concept Introduction:
Material hardness is the property of a metal due to which material resist the plastic deformation. Plastic deformation means material deformation which undergoes non-reversible change. Hardness is the property of any material which process stiffness resistance to bending, scratching or cutting. Hardness is not constant or fixed for all material, but it depends upon strength and plasticity of metal. Material hardness is expressed in terms of hardness number.
Answer to Problem 12.102P
The composition, hardness and amount are
Explanation of Solution
Calculate the amount of martensite from the heating temperature
Therefore at
Applying lever rule at a heating temperature
Put,
Therefore,
The effect of carbon content on the hardness of martensite in steel, the amount of hardness for martensite composition
(d)
Interpretation:
The amount, composition and hardness of material at
Concept Introduction:
Material hardness is the property of a metal due to which material resist the plastic deformation. Plastic deformation means material deformation which undergoes non-reversible change. Hardness is the property of any material which process stiffness resistance to bending, scratching or cutting. Hardness is not constant or fixed for all material, but it depends upon strength and plasticity of metal. Material hardness is expressed in terms of hardness number.
Answer to Problem 12.102P
The composition, hardness and amount are
Explanation of Solution
From the diagram, the eutectoid portion of
r =0.30%c
Applying lever rule, calculate the composition of martensite at them
Calculate the amount of hardness at temperature
From the figure, the effect of carbon content hardness at
Want to see more full solutions like this?
Chapter 12 Solutions
Essentials of Materials Science and Engineering, SI Edition
- Consider the following relational schema and briefly answer the questions that follow: Emp(eid: integer, ename: string, age: integer, salary: real) Works(eid: integer, did: integer, pct_time: integer) Dept(did: integer, budget: real, managerid: integer) a. Define a table constraint on Dept that will ensure that all managers have age > 30. b. Write SQL statements to delete all information about employees whose salaries exceed that of the manager of one or more departments that they work in. Be sure to ensure that all the relevant integrity constraints are satisfied after your updates.arrow_forwardConsider the following relations: Student(snum: integer, sname: string, rmajor: string, level: string, age: integer) Class(cname: string, meets_at: time, room: string, fid: integer) Enrolled(snum: integer, cname: string) Faculty(fid: integer, fname: string, deptid: integer) The meaning of these relations is straightforward; for example, Enrolled has one record per student-class pair such that the student is enrolled in the class. 2. Express each of the following integrity constraints in SQL unless it is implied by the primary and foreign key constraint; if so, explain how it is implied. If the constraint cannot be expressed in SQL, say so. For each constraint, state what operations (inserts, deletes, and updates on specific relations) must be monitored to enforce the constraint. (a) Every faculty member must teach at least two courses. (b) Every student must be enrolled in the course called 'Math101'. (c) A student cannot add more than two courses at a time…arrow_forwardConsider the following relational schema. An employee can work in more than one department; the pct_time field of the Works relation shows the percentage of time that a given employee works in a given department. Emp(eid: integer, ename: string, age: integer, salary: real) Works(eid: integer, did: integer, pct_time: integer) Dept(did: integer, budget: real, managerid: integer) Write the following queries in SQL: a. Print the name of each employee whose salary exceeds the budget of all of the departments that he or she works in. b. Find the enames of managers who manage only departments with budgets larger than $1 million, but at least one department with budget less than $5 million.arrow_forward
- Consider the following schema: Suppliers(sid: integer, sname: string, address: string) Parts(pid: integer, pname: string, color: string) Catalog(sid: integer, pid: integer, cost: real) The Catalog relation lists the prices charged for parts by suppliers. Write the following queries in SQL: a. Find the sids of suppliers who charge more for some part than the average cost of that part (averaged over all the suppliers who supply that part). b. Find the sids of suppliers who supply a red part or a green part. c. For every supplier that supplies a green part and a red part, print the name and price of the most expensive part that she supplies.arrow_forwardThe following relations keep track of airline flight information: Flights(flno: integer, from: string, to: string, distance: integer, departs: time, arrives: time, price: integer) Aircraft(aid: integer, aname: string, cruisingrange: integer) Certified(eid: integer, aid: integer) Employees(eid: integer, ename: string, salary: integer) Note that the Employees relation describes pilots and other kinds of employees as well; every pilot is certified for some aircraft, and only pilots are certified to fly. Write each of the following queries in SQL.(Additional queries using the same schema are listed in the exercises for Chapter 4) a. Identify the routes that can be piloted by every pilot who makes more than $100,000. b. Print the name and salary of every nonpilot whose salary is more than the average salary for pilots. c. Print the names of employees who are certified only on aircrafts with cruising range longer than 1000 miles and who are certified on some Boeing…arrow_forwardDevelop a signal design and timing for the intersection shown in the figure below. In each case accommodate both vehicular and pedestrian movements. In general, use the following values for the problem: pedestrian walking speed = 1 [m/s], vehicle deceleration = 3 [m/s²], driver reaction time = 1.5 [s], length of vehicle = 6 [m], and level grade = 0. If you need to assume other variables and parameters to solve this problem clearly state that in your report and explain the reason. = 4250 1100 70 1100 80 One-way ' 1 900 Speed limit = 50 [km/h] Pedestrian = 15 per each crosswalk Crosswalk widths = 3 [m] Lane width = 4 [m] Saturation flow = 1800 [veh/h/lane] 200arrow_forward
- Qu 1 If crank OA rotates with an angular velocity of ω = 12 rad/s, determine the velocity of piston B and the angular velocity of rod AB at the instant shown. please show all workarrow_forwardQ2/ Maria has an online shop where she sells hand made paintings and cards. She sells the painting for 50 and the card for 20. It takes her 2 hours to complete 1 painting and 45 minutes to make a single card. She also has a day job and makes paintings and cards in her free time. She cannot spend more than 15 hours a week to make paintings and cards. Additionally, she should make not more than 10 paintings and cards per week. She makes a profit of 25 on painting and 15 on each card. How many paintings and cards should she make each week to maximize her profit.arrow_forwardPlease no AI response.arrow_forward
- I have uploaded the rules, please explain step by step and which rule you have appliedarrow_forwardpls match the ans key, someone gave me a wrong ans.arrow_forwardDraw the network diagram of the following Table using PERT and determine Te, Sd, V, ES, LF, Ts and C. P. Find the probability P if D =27 weeks. Activities Prec. by To Tm Tp Te SD V ES LF Ts C.P A -- HW2 11 22U118243 10 10 1985762323443 26624452-2232 B A C -- D C E D F D G F H B, E 1 I G, H J B K J L K, I 4654arrow_forward
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





