
Concept explainers
(a)
Interpretation: The structure of the product formed from acid-catalyzed hydration of
Concept introduction: Acid-catalyzed hydration is the electrophilic addition of water. The reactive species that act as a catalyst is
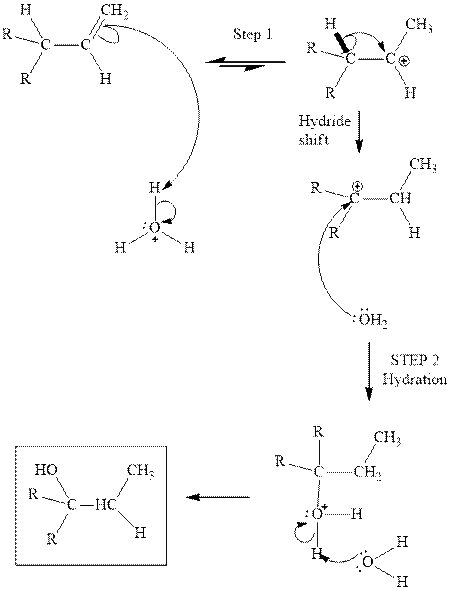
(b)
Interpretation: The structures of isomeric alcohols with chemical formula
Concept introduction: Acid-catalyzed hydration is the electrophilic addition of water. The reactive species that act as a catalyst is
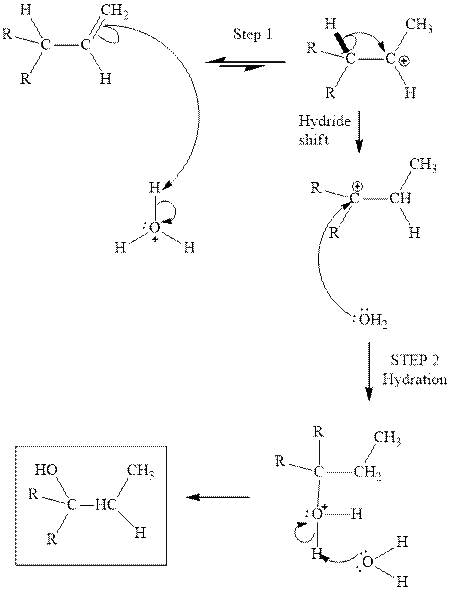
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Experimental Organic Chemistry: A Miniscale & Microscale Approach (Cengage Learning Laboratory Series for Organic Chemistry)
- AE>AE₁ (Y/N) AE=AE₁ (Y/N) AEarrow_forwardTreatment of 2-phenylpropan-2-amine with methyl 2,4-dibromobutanoate in the presence of a nonnucleophilic base, R3N, involves two successive SN2 reactions and gives compound A. ? NH2 Br Br Propose a structural formula for compound A. You do not have to explicitly draw H atoms. You do not have to consider stereochemistry. In cases where there is more than one answer, just draw one. R3N C14H19NO2 + 2 R3NH*Br Aarrow_forwardCorrectly name this compound using the IUPAC naming system by sorting the components into the correct order. Br IN Ν Harrow_forwardHow is the radical intermediate for this structure formed? Can you please draw arrows from the first radical to the resonance form that would result in this product? I'm lost.arrow_forwardPart VI. (a) calculate the λ max of the compound using woodward - Fieser rules. (b) what types of electronic transitions are present in the compound? (c) what are the prominent peaks in the IR spectrum of the compound?arrow_forwardDon't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forward↑ 0 Quiz List - RCC430M_RU05 X Aktiv Learning App × Qdraw resonance structure ×Q draw resonance structure xb My Questions | bartleby ×+ https://app.aktiv.com Draw a resonance structure of pyrrole that has the same number of pi bonds as the original structure. Include all lone pairs in your structure. + N H a 5 19°F Cloudy Q Search Problem 12 of 15 Atoms, Bonds and Rings Charges and Lone Pairs myhp हजु Undo Reset Remove Done Submit Drag To Pan 2:15 PM 1/25/2025arrow_forwardDon't used hand raitingarrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

