
Concept explainers
(a)
Interpretation:Whether the major product formed will have like or unlike stereochemistry or whether it is chiral or meso should be determined.

Concept introduction:In order to assign absolute configuration of R and S, Cahn − Ingold − Prelog rules are used and the first step is to assign the priority order with the
In order to distinguish the relative stereochemistry of two chiral centers situated adjacent to one another, like or unlike notation is used.
For enantiomeric pairs that have same stereochemistry at adjacent carbon atoms, the notation “l” designates “like” and notation “u” designates“un-like”.
Four kinds of symmetry elements that may be present are tabulated as follows:
Presence of any symmetry element makes molecule achiral and optically inactive.
(a)
Explanation of Solution
If the groups now are arranged are read from highest towards least in clockwise fashion then R is assigned to the stereocenter, if the rotation is anticlockwise then S is assigned at the configuration.
The products formed in reaction with bromine are both R due to the clockwise priority order indicated in the reaction below.
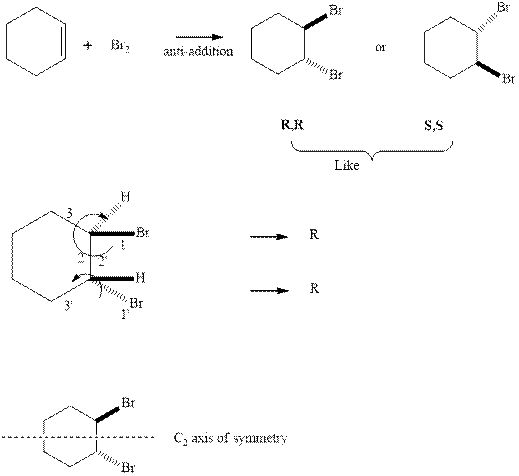
Since the enantiomers have same stereochemistry on both the carbons so they are like. These enantiomers have
(b)
Interpretation:Whether the major product formed will have like or unlike stereochemistry or whether it is chiral or meso should be determined.
Concept introduction:In order to assign absolute configuration of R and S, Cahn − Ingold − Prelog rules are used and the first step is to assign the priority order with the atomic number as the fundamental property. The one with the highest atomic number gest highest priority and is designated as “1” and so on.
In order to distinguish the relative stereochemistry of two chiral centers situated adjacent to one another, like or unlike notation is used.
For enantiomeric pairs that have same stereochemistry at adjacent carbon atoms, the notation “l” designates “like” and notation “u” designates “un-like”.
Four kinds of symmetry elements that may be present are tabulated as follows:
Presence of any symmetry element makes molecule achiral and optically inactive.
(b)
Explanation of Solution
If the groups now are arranged are read from highest towards least in clockwise fashion the R is assigned to the stereocenter, if the rotation is anticlockwise the S is assigned at the configuration.
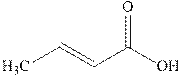
The products formed in the reaction of bromine are R and S indicated in the reaction below.
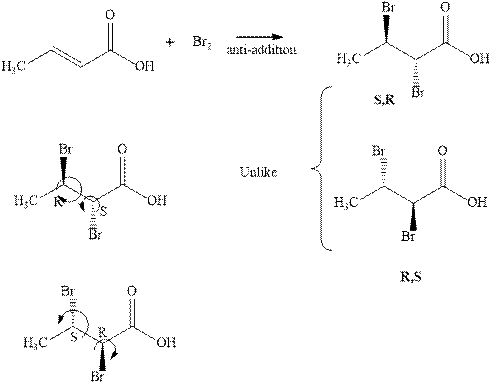
Since the enantiomers have different stereochemistry on both the carbons so they are unlike. These enantiomers have no plane or axis of symmetry so they are chiral.
(c)
Interpretation:Whether the major product formed will have like or unlike stereochemistry or whether it is chiral or meso should be determined.
Concept introduction:In order to assign absolute configuration of R and S, Cahn − Ingold − Prelog rules are used and the first step is to assign the priority order with the atomic number as the fundamental property. The one with the highest atomic number gest highest priority and is designated as “1” and so on.
In order to distinguish the relative stereochemistry of two chiral centers situated adjacent to one another, like or unlike notation is used.
For enantiomeric pairs that have same stereochemistry at adjacent carbon atoms, the notation “l” designates “like” and notation “u” designates “un-like”.
Four kinds of symmetry elements that may be present are tabulated as follows:
Presence of any symmetry element makes molecule achiral and optically inactive.
(c)
Explanation of Solution
If the groups now are arranged are read from highest towards least in clockwise fashion the R is assigned to the stereocenter, if the rotation is anticlockwise the S is assigned at the configuration.
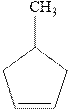
The products formed in the reaction of bromine with given
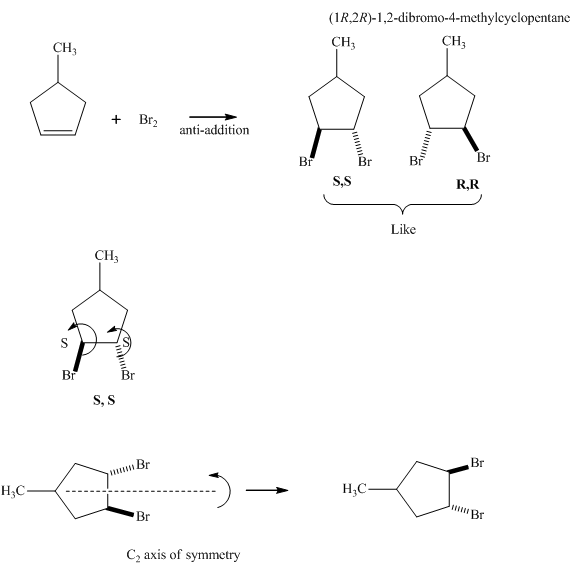
Since the enantiomers have the same stereochemistry on both the carbons so they are like. Further,the presence of
(d)
Interpretation:Whether the major product formed will have like or unlike stereochemistry or whether it is chiral or meso should be determined.

Concept introduction:In order to assign absolute configuration of R and S, Cahn − Ingold − Prelog rules are used and the first step is to assign the priority order with the atomic number as the fundamental property. The one with the highest atomic number gest highest priority and is designated as “1” and so on.
In order to distinguish the relative stereochemistry of two chiral centers situated adjacent to one another, like or unlike notation is used.
For enantiomeric pairs that have same stereochemistry at adjacent carbon atoms, the notation “l” designates “like” and notation “u” designates “un-like”.
Four kinds of symmetry elements that may be present are tabulated as follows:
Presence of any symmetry element makes molecule achiral and optically inactive.
(d)
Explanation of Solution
If the groups now are arranged are read from highest towards least in clockwise fashion the R is assigned to the stereocenter, if the rotation is anticlockwise the S is assigned at the configuration.
The products formed in the reaction of bromine with given alkene are indicated in the reaction below.
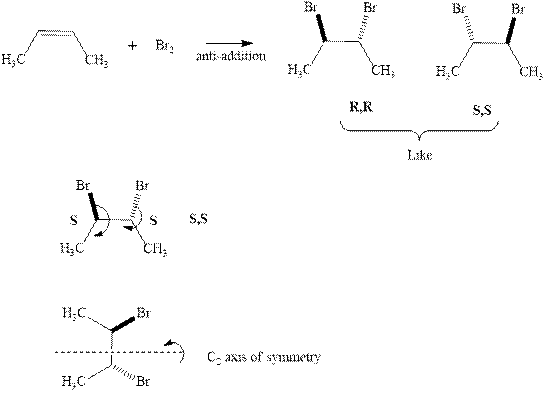
Since the enantiomers have same stereochemistry on both the carbons so they are like. Further presence of
(e)
Interpretation:Whether the major product formed will have like or unlike stereochemistry or whether it is chiral or meso should be determined.

Concept introduction:: In order to assign absolute configuration of R and S, Cahn − Ingold − Prelog rules are used and the first step is to assign the priority order with the atomic number as the fundamental property. The one with the highest atomic number gest highest priority and is designated as “1” and so on.
In order to distinguish the relative stereochemistry of two chiral centers situated adjacent to one another, like or unlike notation is used.
For enantiomeric pairs that have same stereochemistry at adjacent carbon atoms, the notation “l” designates “like” and notation “u” designates “un-like”.
Four kinds of symmetry elements that may be present are tabulated as follows:
Presence of any symmetry element makes molecule achiral and optically inactive.
(e)
Explanation of Solution
If the groups now are arranged are read from highest towards least in clockwise fashion the R is assigned to the stereocenter, if the rotation is anticlockwise the S is assigned at the configuration.
The products formed in the reaction of bromine with given alkene are indicated in the reaction below.
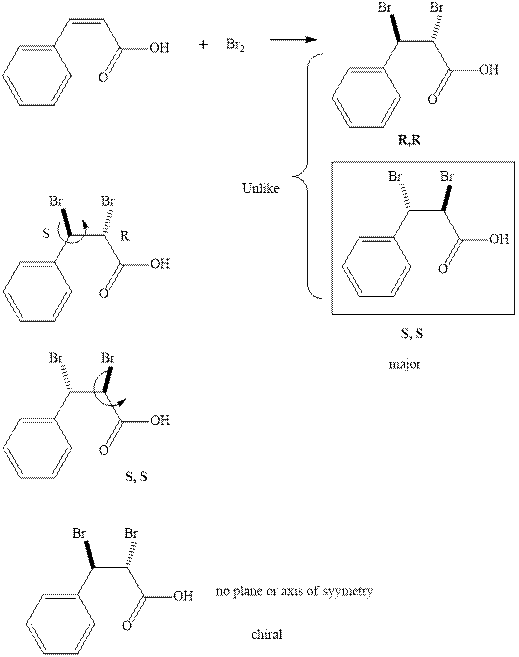
Since the major enantiomer has the same stereochemistry on both the carbons so they are like. These enantiomers have no plane or axis of symmetry so they are chiral.
(f)
Interpretation:Whether the major product formed will have like or unlike stereochemistry or whether it is chiral or meso should be determined.

Concept introduction:: In order to assign absolute configuration of R and S, Cahn − Ingold − Prelog rules are used and the first step is to assign the priority order with the atomic number as the fundamental property. The one with the highest atomic number gest highest priority and is designated as “1” and so on.
In order to distinguish the relative stereochemistry of two chiral centers situated adjacent to one another, like or unlike notation is used.
For enantiomeric pairs that have same stereochemistry at adjacent carbon atoms, the notation “l” designates “like” and notation “u” designates “un-like”.
Four kinds of symmetry elements that may be present are tabulated as follows:
Presence of any symmetry element makes molecule achiral and optically inactive.
(f)
Explanation of Solution
If the groups now are arranged are read from highest towards least in clockwise fashion the R is assigned to the stereocenter, if the rotation is anticlockwise the S is assigned at the configuration.
The products formed in the reaction of bromine with given alkene are R, S and S, R respectively due to the priority order indicated in the reaction below.
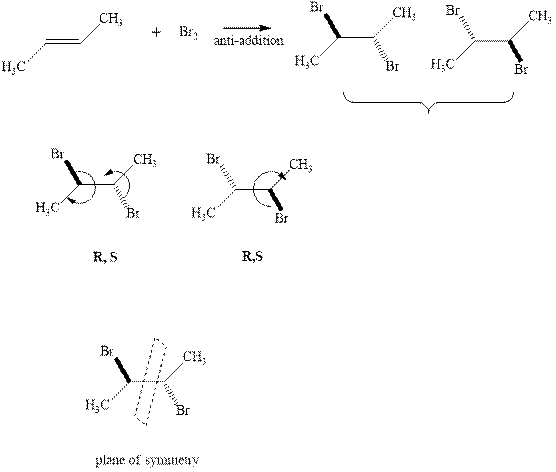
Since the enantiomers have different stereochemistry on both the carbons so they are unlike. These enantiomers havethe plane of symmetry so they are meso.
Want to see more full solutions like this?
Chapter 10 Solutions
Experimental Organic Chemistry: A Miniscale & Microscale Approach (Cengage Learning Laboratory Series for Organic Chemistry)
- In the decomposition reaction in solution B → C, only species C absorbs UV radiation, but neither B nor the solvent absorbs. If we call At the absorbance measured at any time, A0 the absorbance at the beginning of the reaction, and A∞ the absorbance at the end of the reaction, which of the expressions is valid? We assume that Beer's law is fulfilled.arrow_forward> You are trying to decide if there is a single reagent you can add that will make the following synthesis possible without any other major side products: 1. ☑ CI 2. H3O+ O Draw the missing reagent X you think will make this synthesis work in the drawing area below. If there is no reagent that will make your desired product in good yield or without complications, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. Explanation Check ? DO 18 Ar B © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardDon't use ai to answer I will report you answerarrow_forward
- Consider a solution of 0.00304 moles of 4-nitrobenzoic acid (pKa = 3.442) dissolved in 25 mL water and titrated with 0.0991 M NaOH. Calculate the pH at the equivalence pointarrow_forwardWhat is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward
- 7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forwardIndicate the compound formula: dimethyl iodide (propyl) sulfonium.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT




