
(a)
Interpretation: The IR spectra of absorption associated with the C=C bond and trans relationship vinylic H atoms for the spectral data for trans-4-methyl-2-pentene given in the figure needs to be determined.
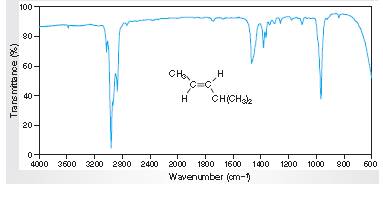
Concept Introduction:
Spectroscopy is the method of identification of structure of the organic molecules with the help of their absorption of certain
(b)
Interpretation The H1 spectra for various resonance to the hydrogen nuclei responsible for them intrans-4-methyl-2-pentene needs to be determined.
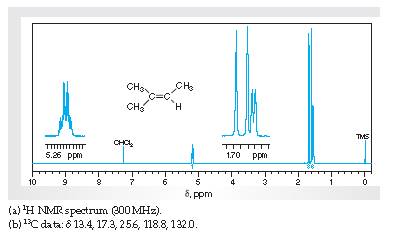
Concept Introduction:
Spectroscopy is the method of identification of structure of the organic molecules with the help of their absorption of certain electromagnetic radiations.
The H1spectroscopy or NMR is based on the nuclear magnetic resonance of radio waves on the H atoms bonded to C atoms present in the organic molecule.
(c)
InterpretationThe C13 spectra for various resonance to the C nuclei responsible for them in trans-4-methyl-2-pentene needs to be determined.
Concept Introduction:
Spectroscopy is the method of identification of structure of the organic molecules with the help of their absorption of certain electromagnetic radiations.
The H1spectroscopy or NMR is based on the nuclear magnetic resonance of radio waves on the H atoms bonded to C atoms present in the organic molecule.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Experimental Organic Chemistry: A Miniscale & Microscale Approach (Cengage Learning Laboratory Series for Organic Chemistry)
- Indicate whether the following two statements are correct or not:- The S8 heterocycle is the origin of a family of compounds- Most of the elements that give rise to stable heterocycles belong to group d.arrow_forwardcould someone draw curly arrow mechanism for this question pleasearrow_forwardIn the phase diagram of quartz (SiO2), indicate what happens as the pressure increases.arrow_forward
- Show work. Don't give Ai generated solutionarrow_forwardNonearrow_forwardTransmitance 3. Which one of the following compounds corresponds to this IR spectrum? Point out the absorption band(s) that helped you decide. OH H3C OH H₂C CH3 H3C CH3 H3C INFRARED SPECTRUM 0.8- 0.6 0.4- 0.2 3000 2000 1000 Wavenumber (cm-1) 4. Consider this compound: H3C On the structure above, label the different types of H's as A, B, C, etc. In table form, list the labeled signals, and for each one state the number of hydrogens, their shifts, and the splitting you would observe for these hydrogens in the ¹H NMR spectrum. Label # of hydrogens splitting Shift (2)arrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
