
Foundations of Materials Science and Engineering
6th Edition
ISBN: 9781259696558
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 8.15, Problem 32AAP
For an alloy of Cu–70 wt% Pb (Fig. 8.24), determine the amounts and compositions in weight percent of the phases present at (a) 955°C + ΔT, (b) 955°C − ΔT, and (c) 200°C.
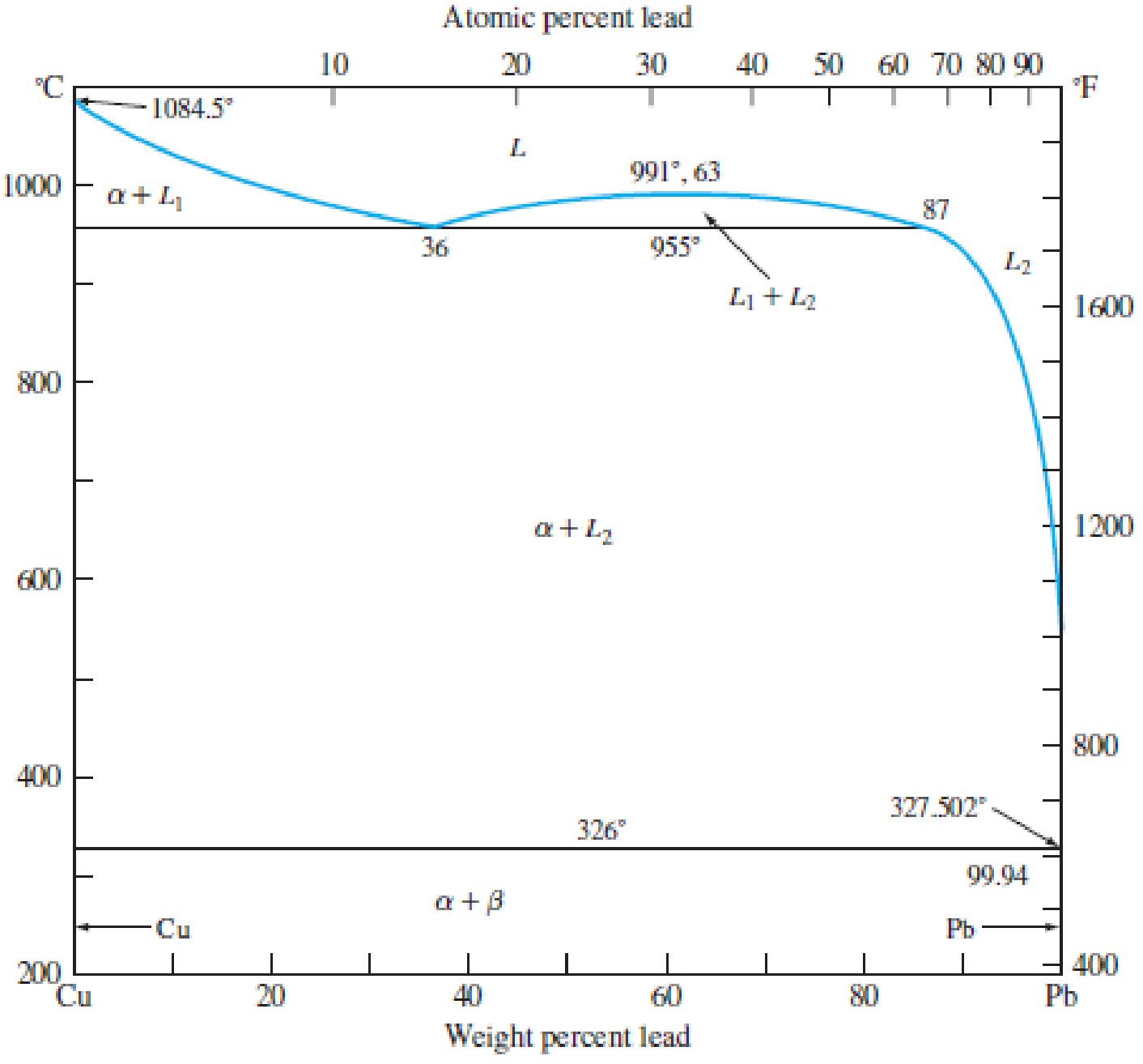
Figure 8.24
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Auto Controls
Perform the partial fraction expansion of the following transfer function and find the impulse response:
G(s) = (s/2 + 5/3) / (s^2 + 4s + 6)
G(s) =( 6s^2 + 50) / (s+3)(s^2 +4)
Study Area
Document Sharing
User Settings
mylabmastering.pearson.com
Access Pearson
P Pearson MyLab and Mastering
The 150-lb skater passes point A with a speed of
6 ft/s. (Figure 1)
Figure
1 of 1
Part A
P Course Home
b My Questions | bartleby
Determine his speed when he reaches point B. Neglect friction.
Express your answer to three significant figures and include the appropriate units.
με
?
VB =
Value
Units
Submit
Request Answer
Part B
Determine the normal force exerted on him by the track at this point.
Express your answer to three significant figures and include the appropriate units.
☐
о
Α
NB =
Value
Units
Submit
Request Answer
Provide Feedback
?
■Review
Next >
mylabmastering.pearson.com
Access Pearson
P Pearson MyLab and Mastering
P Course Home
b My Questions | bartleby
Study Area
Document Sharing
User Settings
The 100-kg crate is subjected to the forces shown. The
crate is originally at rest. The coefficient of kinetic friction
between the crate and the surface is μk = 0.2. (Figure 1)
Part A
Determine the distance it slides in order to attain a speed of 8.1 m/s.
Express your answer to three significant figures and include the appropriate units.
Figure
500 N
1 of 1
Α
S =
Value
Units
Submit
Request Answer
Provide Feedback
?
■Review
Next >
Chapter 8 Solutions
Foundations of Materials Science and Engineering
Ch. 8.15 - Define (a) a phase in a material and (b) a phase...Ch. 8.15 - In the pure water pressure-temperature equilibrium...Ch. 8.15 - How many triple points are there in the pure iron...Ch. 8.15 - Write the equation for the Gibbs phase rule and...Ch. 8.15 - Refer to the pressuretemperature equilibrium phase...Ch. 8.15 - (a) What is a cooling curve? (b) What type of...Ch. 8.15 - Prob. 7KCPCh. 8.15 - What is an alloy? What is the difference between...Ch. 8.15 - Prob. 9KCPCh. 8.15 - What is the significance of the liquidus curve?...
Ch. 8.15 - Prob. 11KCPCh. 8.15 - Prob. 12KCPCh. 8.15 - Prob. 13KCPCh. 8.15 - Describe the mechanism that produces the...Ch. 8.15 - Can coring and surrounding occur in a...Ch. 8.15 - What is a monotectic invariant reaction? How is...Ch. 8.15 - Write equations for the following invariant...Ch. 8.15 - How are eutectic and eutectoid reactions similar?...Ch. 8.15 - Distinguish between (a) a terminal phase and (b)...Ch. 8.15 - Distinguish between (a) an intermediate phase and...Ch. 8.15 - What is the difference between a congruently...Ch. 8.15 - Consider an alloy containing 70 wt% Ni and 30 wt%...Ch. 8.15 - Consider the binary eutectic coppersilver phase...Ch. 8.15 - If 500 g of a 40 wt% Ag60 wt% Cu alloy is slowly...Ch. 8.15 - A lead-tin (PbSn) alloy consists of 60 wt%...Ch. 8.15 - A PbSn alloy (Fig. 8.12) contains 40 wt% and 60...Ch. 8.15 - An alloy of 30 wt% Pb70 wt% Sn is slowly cooled...Ch. 8.15 - Consider the binary peritectic iridiumosmium phase...Ch. 8.15 - Consider the binary peritectic iridiumosmium phase...Ch. 8.15 - Consider the binary peritectic iridiumosmium phase...Ch. 8.15 - In the copperlead (CuPb) system (Fig. 8.24) for an...Ch. 8.15 - For an alloy of Cu70 wt% Pb (Fig. 8.24), determine...Ch. 8.15 - What is the average composition (weight percent)...Ch. 8.15 - Consider an Fe4.2 wt% Ni alloy (Fig. 8.17) that is...Ch. 8.15 - Consider an Fe5.0 wt% Ni alloy (Fig. 8.17) that is...Ch. 8.15 - Determine the weight percent and composition in...Ch. 8.15 - Determine the composition in weight percent of the...Ch. 8.15 - Draw, schematically, the liquidus and the solidus...Ch. 8.15 - Consider the CuZn phase diagram of Figure 8.26. a....Ch. 8.15 - Consider the nickelvanadium phase diagram of...Ch. 8.15 - Consider the titaniumaluminum phase diagram of...Ch. 8.15 - What is the composition of point y in Figure...Ch. 8.15 - In Figure 8.12, determine the degree of freedom,...Ch. 8.15 - The cooling curve of an unknown metal shows a...Ch. 8.15 - In the PbSn phase diagram (Fig. 8.12), answer the...Ch. 8.15 - Based on the CuAg phase diagram in Figure P8.23,...Ch. 8.15 - Based on the PdAg phase diagram in Figure EP 8.3,...Ch. 8.15 - Prob. 49SEPCh. 8.15 - Derive the lever rule for the amount in weight...Ch. 8.15 - Based on the AlNi phase diagram given in Figure...Ch. 8.15 - Prob. 52SEPCh. 8.15 - Based on the Al2O3SiO2 phase diagram in Figure...Ch. 8.15 - (a) Design a CuNi alloy that will be completely...Ch. 8.15 - Prob. 55SEPCh. 8.15 - Given that Pb and Sn have similar tensile...Ch. 8.15 - Consider the sugarwater phase diagram shown in...Ch. 8.15 - In Figure P8.57, if 60 g of water and 140 g of...Ch. 8.15 - In Figure P8.57, if 30 g of water and 170 g of...Ch. 8.15 - At 80C, if the wt% of sugar is 80%, (a) what...Ch. 8.15 - (a) Based on the phase diagram in Figure P8.61,...Ch. 8.15 - Referring to Figure P8.61. explain what happens as...Ch. 8.15 - Referring to Figure P8.61, (a) explain what...Ch. 8.15 - Using Figure P8.40, explain what the phase diagram...Ch. 8.15 - Using Figure P8.40. explain why, according to the...Ch. 8.15 - (a) In the TiAl phase diagram. Figure P8.42, what...Ch. 8.15 - Draw an approximate hypothetical phase diagram for...Ch. 8.15 - Draw the hypothetical phase diagram for a binary...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- The differential equation of a DC motor can be described by the following equation Find the transfer function between the applied voltage ( Va)and the motor speed (thetadot m). What is the steady state speed of the motor after a voltage (Va = 10V) has been applied. Find the transfer function between the applied voltage (Va) and the shaft angle (thetadot m) .arrow_forwardStudy Area Document Sharing User Settings Access Pearson mylabmastering.pearson.com P Pearson MyLab and Mastering The crash cushion for a highway barrier consists of a nest of barrels filled with an impact-absorbing material. The barrier stopping force is measured versus the vehicle penetration into the barrier. (Figure 1) Part A P Course Home b My Questions | bartleby Review Determine the distance a car having a weight of 4000 lb will penetrate the barrier if it is originally traveling at 55 ft/s when it strikes the first barrel. Express your answer to three significant figures and include the appropriate units. Figure 1 of 1 36 μΑ S = Value Units Submit Request Answer Provide Feedback ? Next >arrow_forwardStudy Area Document Sharing User Settings mylabmastering.pearson.com Access Pearson P Pearson MyLab and Mastering Part A P Course Home b My Questions | bartleby ■Review The sports car has a mass of 2.5 Mg and accelerates at 6 m/s², starting from rest. (Figure 1) If the drag resistance on the car due to the wind is FD = (10v) N, where v is the velocity in m/s, determine the power supplied to the engine when t = 5 s. The engine has a running efficiency of € = 0.66. Express your answer to three significant figures and include the appropriate units. Figure 1 of 1 о Α ? P = Value Units Submit Request Answer Return to Assignment Provide Feedbackarrow_forward
- Access Pearson Study Area mylabmastering.pearson.com P Pearson MyLab and Mastering Document Sharing User Settings The car in (Figure 1) having a mass of 2 Mg is originally traveling at 2 m/s. Assume 0 = 22°. Figure 1 of 1 Part A P Course Home b My Questions | bartleby ■Review Determine the distance it must be towed by a force F = 4 kN in order to attain a speed of 6 m/s. Neglect friction and the mass of the wheels. Express your answer to three significant figures and include the appropriate units. Α ? S = Value Units Submit Request Answer Provide Feedback Next >arrow_forwardDerive the Laplace transform of the following functions. Use the definition of Laplace transform. f(t)=sin4t and f(t)=cos2t Auto Controlsarrow_forwardStudy Area Document Sharing User Settings Access Pearson P Pearson MyLab and Mastering Marbles having a mass of 5 g fall from rest at A through the glass tube and accumulate in the can at C. (Figure 1) Figure Aarrow_forward
- VC Vc B S TDC -BDC S TQ Tp = Pg A (asne) [1+ % CUSA] At what position (in degrees after top dead center) would you want the peak pressure of combustion to occur to create the maximum torque on the crankshaft? For a 100mm piston digimeter acting on a connecting. rod with a length of 80mm use the equation above to calculate the torque (NIM) on the crankshaft at this crank position for an engine that develops a peak pressure of 135 bararrow_forwardAccess Pearson P Pearson MyLab and Mastering Study Area Document Sharing User Settings The man having a weight of 180 lb is able to run up a 18-ft-high flight of stairs shiwn in (Figure 1) in 4 s. Figure 1 of 1 R mylabmastering.pearson.com Part A P Course Home b My Questions | bartleby Determine the power generated. Express your answer in horsepower to three significant figures. ΜΕ ΑΣΦ. Η vec P = Submit Request Answer Part B ? hp How long would a 100-W light bulb have to burn to expend the same amount of energy? Express your answer to three significant figures and include the appropriate units. HÅ ? t = Value Units Submit Request Answer Provide Feedback Review Next >arrow_forwardThe tension in the belt is 46 lb. Determine the moment of the force F1 about the pin at A. Determine the moment of the force F2 about the pin at A.arrow_forward
- 1. Describe each of the tolerances in the following drawing: 0.01 A 09±0.025 .10±0.01 0.015 AB 6.76 08.51 03±0.05 0.015 MAB 14±0.03 60 14±0.02 12±0.08 0.01 A Barrow_forwardwhat is the intake flow in cfm of a 5.3 liter engine running at 6200 RPM with a volumetric efficiency of 86%. If we supercharge it to flow 610 CFM what is the volumetric efficiency?arrow_forwardQuiz/An eccentrically loaded bracket is welded to the support as shown in Figure below. The load is static. The weld size for weld w1 is h1=6mm, for w2 h2 5mm, and for w3 is h3 -5.5 mm. Determine the safety factor (S.f) for the welds. F=22 kN. Use an AWS Electrode type (E90xx). 140 101.15 REDMI NOTE 8 PRO AI QUAD CAMERA Farrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Intro to Ceramics and Glasses — Lesson 2, Part 1; Author: Ansys Learning;https://www.youtube.com/watch?v=ArDFnBWH-8w;License: Standard Youtube License