
Foundations of Materials Science and Engineering
6th Edition
ISBN: 9781259696558
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 8.15, Problem 23AAP
Consider the binary eutectic copper–silver phase diagram in Figure P8.23. Make phase analyses of an 88 wt% Ag–12 wt% Cu alloy at the temperatures (a) 1000°C, (b) 800°C, (c) 780°C + ΔT, and (d) 780°C − ΔT. In the phase analyses, include:
- i. The phases present
- ii. The chemical compositions of the phases
- iii. The amounts of each phase
- iv. Sketch the microstructure by using 2-cm-diameter circular fields.
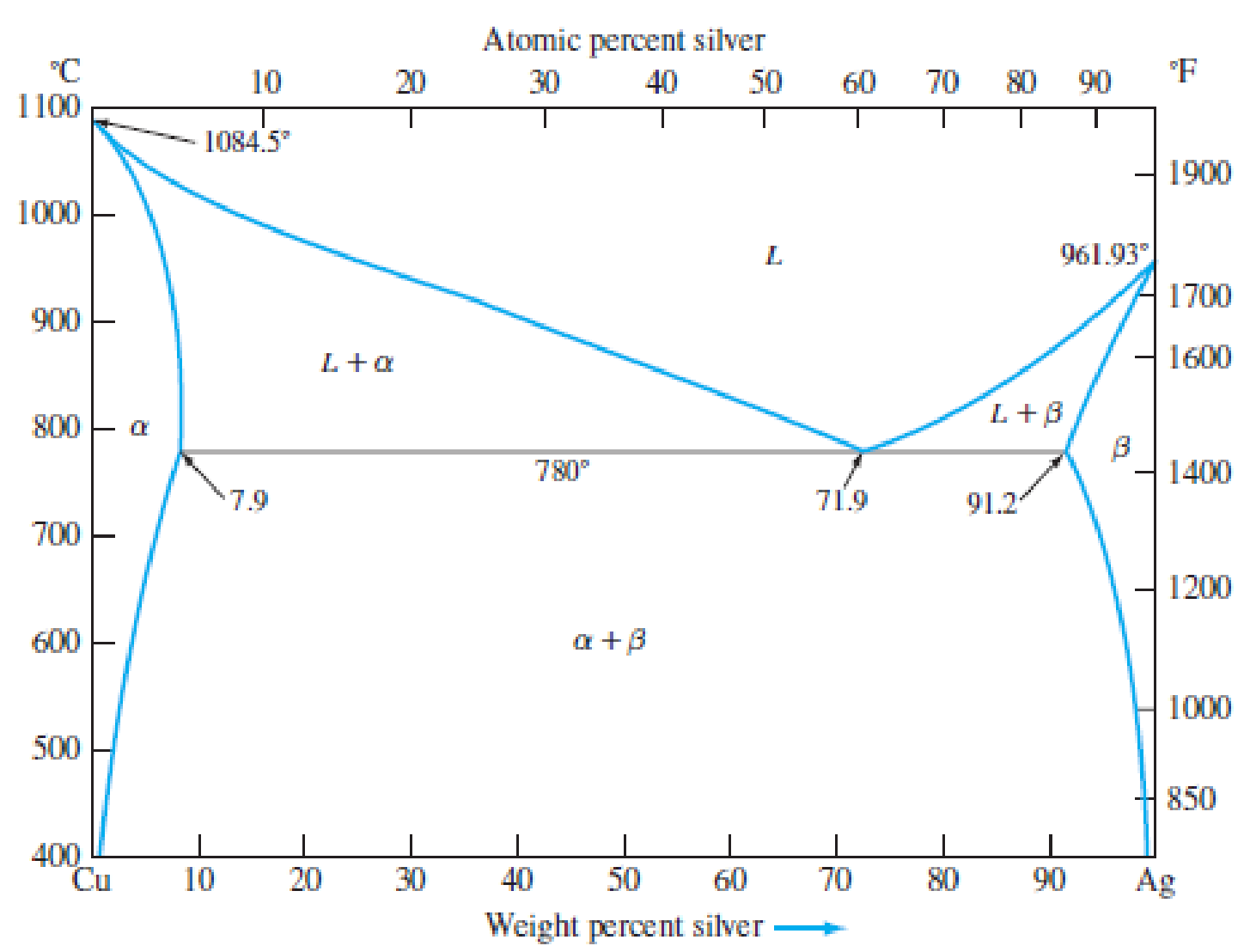
Figure P8.23
The copper–silver phase diagram.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
turbomachienery
auto controls
auto controls
Chapter 8 Solutions
Foundations of Materials Science and Engineering
Ch. 8.15 - Define (a) a phase in a material and (b) a phase...Ch. 8.15 - In the pure water pressure-temperature equilibrium...Ch. 8.15 - How many triple points are there in the pure iron...Ch. 8.15 - Write the equation for the Gibbs phase rule and...Ch. 8.15 - Refer to the pressuretemperature equilibrium phase...Ch. 8.15 - (a) What is a cooling curve? (b) What type of...Ch. 8.15 - Prob. 7KCPCh. 8.15 - What is an alloy? What is the difference between...Ch. 8.15 - Prob. 9KCPCh. 8.15 - What is the significance of the liquidus curve?...
Ch. 8.15 - Prob. 11KCPCh. 8.15 - Prob. 12KCPCh. 8.15 - Prob. 13KCPCh. 8.15 - Describe the mechanism that produces the...Ch. 8.15 - Can coring and surrounding occur in a...Ch. 8.15 - What is a monotectic invariant reaction? How is...Ch. 8.15 - Write equations for the following invariant...Ch. 8.15 - How are eutectic and eutectoid reactions similar?...Ch. 8.15 - Distinguish between (a) a terminal phase and (b)...Ch. 8.15 - Distinguish between (a) an intermediate phase and...Ch. 8.15 - What is the difference between a congruently...Ch. 8.15 - Consider an alloy containing 70 wt% Ni and 30 wt%...Ch. 8.15 - Consider the binary eutectic coppersilver phase...Ch. 8.15 - If 500 g of a 40 wt% Ag60 wt% Cu alloy is slowly...Ch. 8.15 - A lead-tin (PbSn) alloy consists of 60 wt%...Ch. 8.15 - A PbSn alloy (Fig. 8.12) contains 40 wt% and 60...Ch. 8.15 - An alloy of 30 wt% Pb70 wt% Sn is slowly cooled...Ch. 8.15 - Consider the binary peritectic iridiumosmium phase...Ch. 8.15 - Consider the binary peritectic iridiumosmium phase...Ch. 8.15 - Consider the binary peritectic iridiumosmium phase...Ch. 8.15 - In the copperlead (CuPb) system (Fig. 8.24) for an...Ch. 8.15 - For an alloy of Cu70 wt% Pb (Fig. 8.24), determine...Ch. 8.15 - What is the average composition (weight percent)...Ch. 8.15 - Consider an Fe4.2 wt% Ni alloy (Fig. 8.17) that is...Ch. 8.15 - Consider an Fe5.0 wt% Ni alloy (Fig. 8.17) that is...Ch. 8.15 - Determine the weight percent and composition in...Ch. 8.15 - Determine the composition in weight percent of the...Ch. 8.15 - Draw, schematically, the liquidus and the solidus...Ch. 8.15 - Consider the CuZn phase diagram of Figure 8.26. a....Ch. 8.15 - Consider the nickelvanadium phase diagram of...Ch. 8.15 - Consider the titaniumaluminum phase diagram of...Ch. 8.15 - What is the composition of point y in Figure...Ch. 8.15 - In Figure 8.12, determine the degree of freedom,...Ch. 8.15 - The cooling curve of an unknown metal shows a...Ch. 8.15 - In the PbSn phase diagram (Fig. 8.12), answer the...Ch. 8.15 - Based on the CuAg phase diagram in Figure P8.23,...Ch. 8.15 - Based on the PdAg phase diagram in Figure EP 8.3,...Ch. 8.15 - Prob. 49SEPCh. 8.15 - Derive the lever rule for the amount in weight...Ch. 8.15 - Based on the AlNi phase diagram given in Figure...Ch. 8.15 - Prob. 52SEPCh. 8.15 - Based on the Al2O3SiO2 phase diagram in Figure...Ch. 8.15 - (a) Design a CuNi alloy that will be completely...Ch. 8.15 - Prob. 55SEPCh. 8.15 - Given that Pb and Sn have similar tensile...Ch. 8.15 - Consider the sugarwater phase diagram shown in...Ch. 8.15 - In Figure P8.57, if 60 g of water and 140 g of...Ch. 8.15 - In Figure P8.57, if 30 g of water and 170 g of...Ch. 8.15 - At 80C, if the wt% of sugar is 80%, (a) what...Ch. 8.15 - (a) Based on the phase diagram in Figure P8.61,...Ch. 8.15 - Referring to Figure P8.61. explain what happens as...Ch. 8.15 - Referring to Figure P8.61, (a) explain what...Ch. 8.15 - Using Figure P8.40, explain what the phase diagram...Ch. 8.15 - Using Figure P8.40. explain why, according to the...Ch. 8.15 - (a) In the TiAl phase diagram. Figure P8.42, what...Ch. 8.15 - Draw an approximate hypothetical phase diagram for...Ch. 8.15 - Draw the hypothetical phase diagram for a binary...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 1 Pleasearrow_forwardA spring cylinder system measures the pressure. Determine which spring can measure pressure between 0-1 MPa with a large excursion. The plate has a diameter of 20 mm. Also determine the displacement of each 0.1 MPa step.Spring power F=c x fF=Springpower(N)c=Spring constant (N/mm)f=Suspension (mm) How do I come up with right answer?arrow_forwardA lift with a counterweight is attached to the ceiling. The attachment is with 6 stainless and oiled screws. What screw size is required? What tightening torque? - The lift weighs 500 kg and can carry 800 kg. - Counterweight weight 600 kg - Durability class 12.8 = 960 MPa- Safety factor ns=5+-Sr/Fm= 0.29Gr =0.55arrow_forward
- Knowing that a force P of magnitude 750 N is applied to the pedal shown, determine (a) the diameter of the pin at C for which the average shearing stress in the pin is 40 MPa, (b) the corresponding bearing stress in the pedal at C, (c) the corresponding bearing stress in each support bracket at C. 75 mm 300 mm- mm A B P 125 mm 5 mm C Darrow_forwardAssume the B frame differs from the N frame through a 90 degree rotation about the second N base vector. The corresponding DCM description is: 1 2 3 4 5 6 9 # adjust the return matrix values as needed def result(): dcm = [0, 0, 0, 0, 0, 0, 0, 0, 0] return dcmarrow_forwardFind the reaction at A and B The other response I got was not too accurate,I need expert solved answer, don't use Artificial intelligence or screen shot it solvingarrow_forward
- A six cylinder petrol engine has a compression ratio of 5:1. The clearance volume of each cylinder is 110CC. It operates on the four-stroke constant volume cycle and the indicated efficiency ratio referred to air standard efficiency is 0.56. At the speed of 2400 rpm. 44000KJ/kg. Determine the consumes 10kg of fuel per hour. The calorific value of fuel average indicated mean effective pressure.arrow_forwardThe members of a truss are connected to the gusset plate as shown in (Figure 1). The forces are concurrent at point O. Take = 90° and T₁ = 7.5 kN. Part A Determine the magnitude of F for equilibrium. Express your answer to three significant figures and include the appropriate units. F= 7.03 Submit ? kN Previous Answers Request Answer × Incorrect; Try Again; 21 attempts remaining ▾ Part B Determine the magnitude of T2 for equilibrium. Express your answer to three significant figures and include the appropriate units. Figure T₂ = 7.03 C T2 |? KN Submit Previous Answers Request Answer × Incorrect; Try Again; 23 attempts remaining Provide Feedbackarrow_forwardConsider the following acid-base reaction: Fe3+(aq) +3H2O -Fe(OH)3 (s) + 3H* ← A. Using thermodynamics, calculate the equilibrium constant K at 25°C (The AG° of formation of Fe(OH)3(s) is -699 kJ/mol). B. Using the value of K you calculated in part a, if a solution contains 10-4 M Fe3+ and has a pH of 7.5, will Fe(OH)3(s) precipitate? Show all calculations necessary to justify your answer. Note that the reaction as written is for precipitation, not dissolution like Ksp-arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Material Science, Phase Diagrams, Part 1; Author: Welt der Werkstoffe;https://www.youtube.com/watch?v=G83ZaoB3XCc;License: Standard Youtube License