
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 17E
Build a model of methylcyclohexane, and use the model to complete the following Newmanprojections of methylcyclohexane in the chair conformation: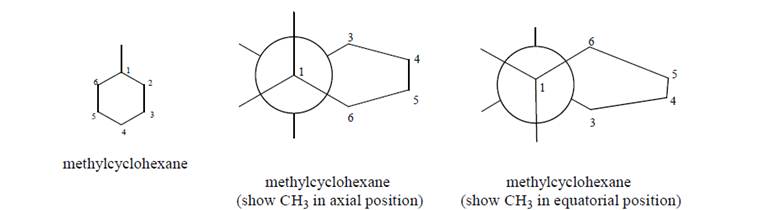 a. When the methyl group is in an axial or equatorial (circle one) position, the molecule is inits lowest potential energy conformation.
a. When the methyl group is in an axial or equatorial (circle one) position, the molecule is inits lowest potential energy conformation.
b. Label one Newman projection above anti and the other gauche to describe the relationshipbetween the methyl group and
c. In general, which is a lower PE conformation, anti or gauche?
d. Explain how your answer to b and c provide an explanation for why it is more favorable fora large group to be in an equatorial than an axial position.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Predict the major products of the following organic reaction:
Some important notes:
Δ
CN
?
• Draw the major product, or products, of the reaction in the drawing area below.
• If there aren't any products, because no reaction will take place, check the box below the drawing area instead.
Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are
enantiomers.
ONO reaction.
Click and drag to start drawing a structure.
The following product was made from diethyl ketone and what other reagent(s)?
£
HO
10
2-pentyne
1-butyne and NaNH2
☐ 1-propanol
☐ pyridine
butanal
☐ pentanoate
Which pair of reagents will form the given product?
OH
X
+
Y
a.
CH3
b.
CH2CH3
༧་་
C. CH3-
CH2CH3
d.o6.(རི॰
e.
CH3
OCH2CH3
-MgBr
f. CH3-MgBr
g. CH3CH2-MgBr
-C-CH3
CH2CH3
Chapter 7 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 7 - Prob. 1CTQCh. 7 - Prob. 2CTQCh. 7 - Prob. 3CTQCh. 7 - Draw wedge and dash skeletal representations of...Ch. 7 - Label each ring in Figure 7.2 cis or trans.Ch. 7 - Prob. 6CTQCh. 7 - Prob. 7CTQCh. 7 - Prob. 8CTQCh. 7 - a model of cyclohexane in a chair conformation,...Ch. 7 - Prob. 10CTQ
Ch. 7 - Prob. 11CTQCh. 7 - Fill in the blanks: cis-1,3-Dimethylcyclohexane...Ch. 7 - Prob. 13CTQCh. 7 - Prob. 14CTQCh. 7 - Prob. 15CTQCh. 7 - Prob. 16CTQCh. 7 - Prob. 17CTQCh. 7 - Prob. 18CTQCh. 7 - Draw chair representations of...Ch. 7 - Which stereoisomer in the previous question is...Ch. 7 - Prob. 21CTQCh. 7 - Prob. 1ECh. 7 - Label each of the following as cis, trans or...Ch. 7 - Which pair has more in common with one another?Ch. 7 - Prob. 4ECh. 7 - Prob. 5ECh. 7 - Prob. 6ECh. 7 - Fill in the table by drawing a representation of a...Ch. 7 - Prob. 9ECh. 7 - True or False: If you perform a chair flip on...Ch. 7 - Prob. 11ECh. 7 - Prob. 12ECh. 7 - Prob. 13ECh. 7 - Prob. 14ECh. 7 - Prob. 15ECh. 7 - Draw trans-1-tert-butyl-3-methylcyclohexane in its...Ch. 7 - Build a model of methylcyclohexane, and use the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 3 What best describes the product of the following reaction? 1. CH3CH2MgBr (2 eq) 2. H a new stereocenter will not be formed a new stereocenter will be formed an alkyl halide will result an alkane will result an aromatic compound will result 1 ptsarrow_forwardRank the following from most to least reactive toward nucleophilic attack. 1. [Select] [Select] 2. Acyl halide Aldehyde 3. Carboxylate ion 4. Carboxylic acid Ketone 5. [Select]arrow_forwardQuestion 10 1 pts Which of the following is the most accurate nomenclature? 1-hydroxy-1-methyldecane-4,7-dione 2-hydroxy-2-methyldecane-5,8-dione 4,6-dioxo-2-methyldecane-2-ol 9-hydroxy-9-methyldecane-3,6-dione 8-hydroxy-8-methylnonane-3,6-dione OHarrow_forward
- Could you please explain whether my thinking is correct or incorrect regarding how I solved it? Please point out any mistakes in detail, with illustrations if needed.arrow_forwardWhat are the most proper reagents to achieve these products? سد 1. 2. OH ○ 1. BrMgC6H6; 2. H+ ○ 1. BrMgCH2CH2CH2CH2CH3; 2. H+ O 1. CH3CH2CHO; 2. H+ O 1. BrMgCH2CH3; 2. H+arrow_forwardProvide the IUPAC (systematic) name only for the following compound. Dashes, commas, and spaces must be correct. Harrow_forward
- Please use the nernst equation to genereate the Ion Selective Electrode Analysis standard curve within my excel spread sheet. Nernst Equation: E = Eo + m (ln a) Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EaREe1-PfGNKq1Cbink6kkYB5lBy05hEaE3mbGPUb22S6w?rtime=zQaSX3xY3Ugarrow_forwarda) b) c) H NaOH heat, dehydration + KOH heat, dehydration NaOH + (CH3)3CCHO heat, dehydration Pharrow_forwardshow mechanismarrow_forward
- Please draw by handarrow_forward3. Predict the major product and give a mechanism for the following reactions: (CH3)3COH/H₂SO4 a) b) NC CH₂O c) LOCH, (CH3)3COH/H2SO4 H,SO -OHarrow_forwardIndicate if the aldehyde shown reacts with the provided nucleophiles in acid or base conditions. a NaBH4 be Li eli -NH2 P(Ph3) f KCN g OH excess h CH3OH i NaCHCCH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License