
Concept explainers
Interpretation:Whether in the model of 1,2-dimethylcyclopentane, the molecule in the left box is same as the molecule in the right box or not needs to be determined.
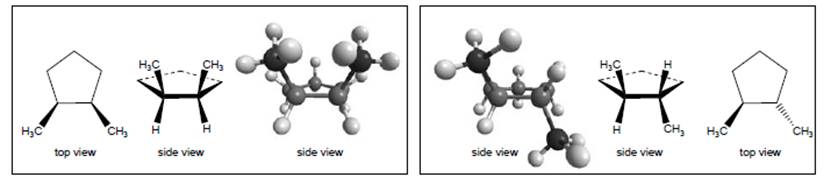
Concept Introduction:
The cis and trans isomerism concept will be applied here. Any molecule will be called cis if it follows the conditions for itthat is the same groups attached either above the plane or below the plane.For trans isomer,same groups are attached where one is above the plane and other group is below the plane or vice versa.
Answer to Problem 1CTQ
The given both structures of isomers of 1,2 dimethylcyclopentane are similar to each other. This is because when the bonds of the groups interchanges with each other, the bond’s spatial character changes. As a result of which these structures can be converted to each other without breaking of bonds.
Explanation of Solution
It is given that the left box and right box contain two molecules of 1,2-dimethylcyclopentane.Thus, it is very easy to conclude that the 2 different molecules are isomers of 1,2-dimethylcyclopentane. This is because both the molecules have same number of atoms in it.
Both the molecules are similar toeach other. Thiscan be explained as, if the interchanging of bonds takes place, similar structure can be obtained. In the interchanging of structure in left box and right box, there is no breaking of bonds takes place. For example, if the methyl group attached to the bond below the planeis interchanged with other group attached to the same atom, then the position of methyl group will be converted to above the plane from below the plane. In this process, no breaking of bond takes place then similar structure will be obtained.
Thus, the structure on the left box is similar to that on the right box.
Want to see more full solutions like this?
Chapter 7 Solutions
Organic Chemistry: A Guided Inquiry
- 8:16 PM Sun Mar 30 K Draw the major product of this reaction. Ignore inorganic byproducts. Proble 1. CH3MgBr 2. H3O+ F Drawingarrow_forwardо но оarrow_forwardName the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forward
- Macmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forwardProvide the reagents for the following reactions.arrow_forward
- If I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forwardPredict the major organic product(s) for the following reaction.arrow_forward
- Predict the major organic product(s) for the following reactions.arrow_forwardProvide the complete mechanism for the reactions below. You must include appropriate arrows,intermediates, and formal charges.arrow_forwardIndicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
