
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 7, Problem 15CTQ
Interpretation Introduction
Interpretation: The structure representing black balls at axial position and at equatorial position needs to be determined.
Concept Introduction: The chair conformation of cyclohexane is represented as follows:

During the flipping, no bond is break. The numbering in the chair form is represented as follows:
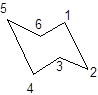
During ring flipping, mirror image of the chair conformation is formed.
It is represented as follows:
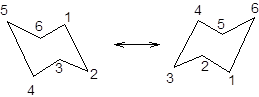
Now, the ring flipping with substituent attached can be represented as follows;
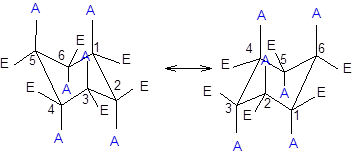
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
6.15PM
Sun Mar 30
K
Draw the major product of this reaction. Include
any relevant stereochemistry. Ignore inorganic
byproducts.
Problem 1 of
O
H
[PhзPCH2CH3]*C|¯
NaH
Drawing
>
Q
Atoms,
Bonds and
Draw or tap a ne
8:17 PM Sun Mar 30
Draw the major product of this reaction. Ignore
inorganic byproducts.
HSCH2CH2CH2SH, BF3
Probler
Drawing
Ato
Bonds
Cl
presented by Mr L
How the coprion. (Il Done in no wraction, dew the starting redential)
до
Chapter 7 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 7 - Prob. 1CTQCh. 7 - Prob. 2CTQCh. 7 - Prob. 3CTQCh. 7 - Draw wedge and dash skeletal representations of...Ch. 7 - Label each ring in Figure 7.2 cis or trans.Ch. 7 - Prob. 6CTQCh. 7 - Prob. 7CTQCh. 7 - Prob. 8CTQCh. 7 - a model of cyclohexane in a chair conformation,...Ch. 7 - Prob. 10CTQ
Ch. 7 - Prob. 11CTQCh. 7 - Fill in the blanks: cis-1,3-Dimethylcyclohexane...Ch. 7 - Prob. 13CTQCh. 7 - Prob. 14CTQCh. 7 - Prob. 15CTQCh. 7 - Prob. 16CTQCh. 7 - Prob. 17CTQCh. 7 - Prob. 18CTQCh. 7 - Draw chair representations of...Ch. 7 - Which stereoisomer in the previous question is...Ch. 7 - Prob. 21CTQCh. 7 - Prob. 1ECh. 7 - Label each of the following as cis, trans or...Ch. 7 - Which pair has more in common with one another?Ch. 7 - Prob. 4ECh. 7 - Prob. 5ECh. 7 - Prob. 6ECh. 7 - Fill in the table by drawing a representation of a...Ch. 7 - Prob. 9ECh. 7 - True or False: If you perform a chair flip on...Ch. 7 - Prob. 11ECh. 7 - Prob. 12ECh. 7 - Prob. 13ECh. 7 - Prob. 14ECh. 7 - Prob. 15ECh. 7 - Draw trans-1-tert-butyl-3-methylcyclohexane in its...Ch. 7 - Build a model of methylcyclohexane, and use the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 8:16 PM Sun Mar 30 K Draw the major product of this reaction. Ignore inorganic byproducts. Proble 1. CH3MgBr 2. H3O+ F Drawingarrow_forwardо но оarrow_forwardName the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forward
- Macmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forwardProvide the reagents for the following reactions.arrow_forward
- If I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forwardPredict the major organic product(s) for the following reaction.arrow_forward
- Predict the major organic product(s) for the following reactions.arrow_forwardProvide the complete mechanism for the reactions below. You must include appropriate arrows,intermediates, and formal charges.arrow_forwardIndicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License