
Concept explainers
(a)
Interpretation: The molecule with the lowest potential energy needs to be drawn for the following condition:
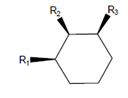
Here,
Concept Introduction: The molecule with lowest potential energy is most stable. In the chair conformation of the cyclohexane, the stable conformation is when large groups are present in the equatorial positions. The equatorial positions in the chair conformation points away from the ring thus, there is less steric hinderance.
(b)
Interpretation: The molecule with the lowest potential energy needs to be drawn for the following condition:
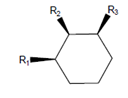
Here,
Concept Introduction: The molecule with lowest potential energy is most stable. In the chair conformation of the cyclohexane, the stable conformation is when large groups are present in the equatorial positions. The equatorial positions in the chair conformation points away from the ring thus, there is less steric hinderance.
(c)
Interpretation: The molecule with the lowest potential energy needs to be drawn for the following condition:
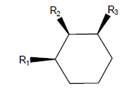
Here,
Concept Introduction: The molecule with lowest potential energy is most stable. In the chair conformation of the cyclohexane, the stable conformation is when large groups are present in the equatorial positions. The equatorial positions in the chair conformation points away from the ring thus, there is less steric hinderance.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry: A Guided Inquiry
- о но оarrow_forwardName the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forwardMacmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forward
- If I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forwardProvide the reagents for the following reactions.arrow_forwardIf I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forwardPredict the major organic product(s) for the following reaction.arrow_forwardPredict the major organic product(s) for the following reactions.arrow_forward
- Provide the complete mechanism for the reactions below. You must include appropriate arrows,intermediates, and formal charges.arrow_forwardIndicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. C6H5 CH3arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
