
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 7, Problem 16CTQ
Interpretation Introduction
Interpretation: In the given structures, each black ball needs to be labeled as up or down.
Concept Introduction: Chair conformation is the most stable conformation of cyclohexane. It is represented as follows:

Here, the substituted groups in the chair conformation are represented as follows:
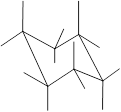
The groups showing in straight upward and downward directions are axial and the groups bend slightly right or left are equatorial.
The axial and equatorial groups are represented in the chair conformation as A and E:
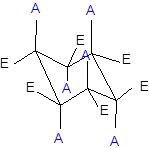
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
6.15PM
Sun Mar 30
K
Draw the major product of this reaction. Include
any relevant stereochemistry. Ignore inorganic
byproducts.
Problem 1 of
O
H
[PhзPCH2CH3]*C|¯
NaH
Drawing
>
Q
Atoms,
Bonds and
Draw or tap a ne
8:17 PM Sun Mar 30
Draw the major product of this reaction. Ignore
inorganic byproducts.
HSCH2CH2CH2SH, BF3
Probler
Drawing
Ato
Bonds
Cl
presented by Mr L
How the coprion. (Il Done in no wraction, dew the starting redential)
до
Chapter 7 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 7 - Prob. 1CTQCh. 7 - Prob. 2CTQCh. 7 - Prob. 3CTQCh. 7 - Draw wedge and dash skeletal representations of...Ch. 7 - Label each ring in Figure 7.2 cis or trans.Ch. 7 - Prob. 6CTQCh. 7 - Prob. 7CTQCh. 7 - Prob. 8CTQCh. 7 - a model of cyclohexane in a chair conformation,...Ch. 7 - Prob. 10CTQ
Ch. 7 - Prob. 11CTQCh. 7 - Fill in the blanks: cis-1,3-Dimethylcyclohexane...Ch. 7 - Prob. 13CTQCh. 7 - Prob. 14CTQCh. 7 - Prob. 15CTQCh. 7 - Prob. 16CTQCh. 7 - Prob. 17CTQCh. 7 - Prob. 18CTQCh. 7 - Draw chair representations of...Ch. 7 - Which stereoisomer in the previous question is...Ch. 7 - Prob. 21CTQCh. 7 - Prob. 1ECh. 7 - Label each of the following as cis, trans or...Ch. 7 - Which pair has more in common with one another?Ch. 7 - Prob. 4ECh. 7 - Prob. 5ECh. 7 - Prob. 6ECh. 7 - Fill in the table by drawing a representation of a...Ch. 7 - Prob. 9ECh. 7 - True or False: If you perform a chair flip on...Ch. 7 - Prob. 11ECh. 7 - Prob. 12ECh. 7 - Prob. 13ECh. 7 - Prob. 14ECh. 7 - Prob. 15ECh. 7 - Draw trans-1-tert-butyl-3-methylcyclohexane in its...Ch. 7 - Build a model of methylcyclohexane, and use the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 8:16 PM Sun Mar 30 K Draw the major product of this reaction. Ignore inorganic byproducts. Proble 1. CH3MgBr 2. H3O+ F Drawingarrow_forwardо но оarrow_forwardName the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forward
- Macmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forwardProvide the reagents for the following reactions.arrow_forward
- If I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forwardPredict the major organic product(s) for the following reaction.arrow_forward
- Predict the major organic product(s) for the following reactions.arrow_forwardProvide the complete mechanism for the reactions below. You must include appropriate arrows,intermediates, and formal charges.arrow_forwardIndicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY