
Pearson eText for Essential Organic Chemistry -- Instant Access (Pearson+)
3rd Edition
ISBN: 9780137533268
Author: Paula Bruice
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6.6, Problem 13P
What stereoisomers are obtained from each of the following reactions?
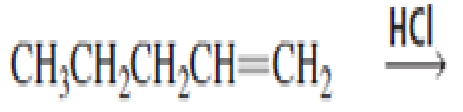
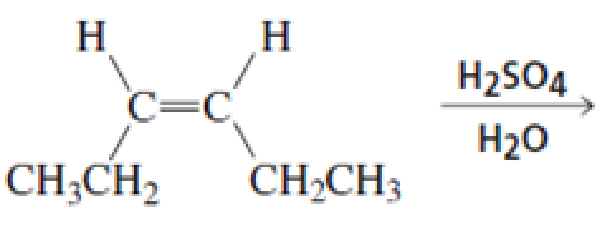
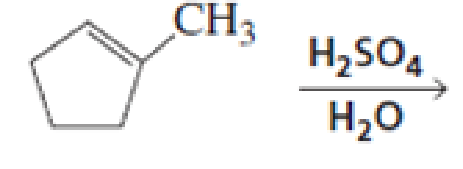
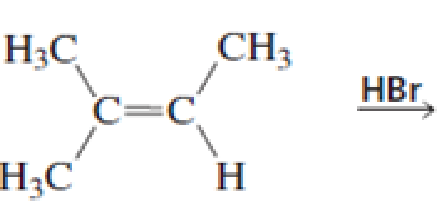
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Part II. Identify whether the two protons in blue are homotopic, enantiopic, diasteriotopic, or heterotopic.
a)
HO
b)
Bri
H
HH
c)
d)
H
H H Br
0
None
Choose the option that is decreasing from biggest to smallest.
Group of answer choices:
100 m, 10000 mm, 100 cm, 100000 um, 10000000 nm
10000000 nm, 100000 um, 100 cm, 10000 mm, 100 m
10000000 nm, 100000 um, 10000 mm, 100 cm, 100 m
100 m, 100 cm, 10000 mm, 100000 um, 10000000 nm
Chapter 6 Solutions
Pearson eText for Essential Organic Chemistry -- Instant Access (Pearson+)
Ch. 6.1 - Draw the mechanism for the reaction of cyclohexene...Ch. 6.2 - a. How many bond orbitals are available for...Ch. 6.2 - Prob. 3PCh. 6.2 - Prob. 4PCh. 6.3 - Prob. 5PCh. 6.3 - Prob. 6PCh. 6.3 - Prob. 7PCh. 6.5 - Prob. 9PCh. 6.5 - Prob. 10PCh. 6.5 - a. What is the major product of each of the...
Ch. 6.5 - Prob. 12PCh. 6.6 - What stereoisomers are obtained from each of the...Ch. 6.6 - Prob. 14PCh. 6.8 - Prob. 15PCh. 6.10 - Name the following:Ch. 6.10 - Draw the structure for each of the following: a....Ch. 6.10 - Draw the structures for and name the seven alkynes...Ch. 6.10 - Name the following:Ch. 6.10 - Name the following:Ch. 6.11 - What hybrid orbitals are used to form the...Ch. 6.13 - Prob. 22PCh. 6.14 - Prob. 23PCh. 6.14 - Which alkyne would be the best one to use for the...Ch. 6.14 - Prob. 25PCh. 6.14 - Prob. 26PCh. 6.15 - Describe the alkyne you would start with and the...Ch. 6.15 - What are products of the following reactions?Ch. 6 - Prob. 29PCh. 6 - Prob. 30PCh. 6 - Prob. 31PCh. 6 - Prob. 32PCh. 6 - What is each compounds systematic name?Ch. 6 - Prob. 34PCh. 6 - Prob. 35PCh. 6 - What reagents could be used to carry out the...Ch. 6 - Prob. 37PCh. 6 - Prob. 38PCh. 6 - Prob. 39PCh. 6 - Prob. 40PCh. 6 - Prob. 41PCh. 6 - Prob. 42PCh. 6 - Answer Problem 42 using 2-butyne as the starting...Ch. 6 - What is each compounds systematic name?Ch. 6 - Prob. 45PCh. 6 - Prob. 46PCh. 6 - Prob. 47PCh. 6 - Prob. 48PCh. 6 - Prob. 49PCh. 6 - Prob. 50PCh. 6 - Draw the keto tautomer for each of the following:Ch. 6 - Propose a mechanism for the following reaction...Ch. 6 - Prob. 53PCh. 6 - Prob. 54PCh. 6 - Prob. 55PCh. 6 - Propose a mechanism for the following reaction:Ch. 6 - Prob. 57PCh. 6 - Prob. 58PCh. 6 - Prob. 59PCh. 6 - Prob. 60PCh. 6 - Prob. 61PCh. 6 - Prob. 62P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q1. (a) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH3. Use curved arrows to show the electron movement. (b) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH4*. Use curved arrows to show the electron movement.arrow_forwardWhich is NOT the typical size of a bacteria? 1000 nm 0.001 mm 0.01 mm 1 umarrow_forwardNonearrow_forward
- Show work. don't give Ai generated solutionarrow_forwardPart II. count the expected number of signals in the 1H-NMR spectrum of these compounds HO 0 одев * Cl -cl "D"arrow_forwardPart I. Create a splitting tree diagram to predict the multiplet pattern of proton Hb in the compound below: 3 (Assume that "Jab >>> ³JbC) Ha Hb He он Ha NH2 Ha HCarrow_forward
- SH 0 iq noitzouDarrow_forwardNonearrow_forward+ HCl →? Draw the molecule on the canvas by choosing buttons from the Tools (for bonas), Atoms and Advanced Template toolbars. The single bond is active by default. + M C + H± 2D EXP. CONT. K ? L 1 H₁₂C [1] A HCN O S CH3 CH 3 CI Br HC H₂ CH CH CH3 - P Farrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License