
Pearson eText for Essential Organic Chemistry -- Instant Access (Pearson+)
3rd Edition
ISBN: 9780137533268
Author: Paula Bruice
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6.11, Problem 21P
What hybrid orbitals are used to form the carbon-carbon
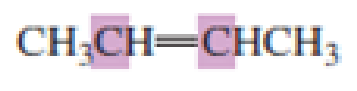


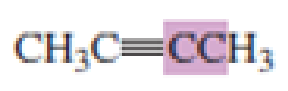

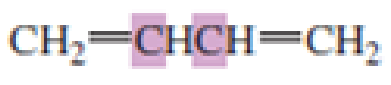

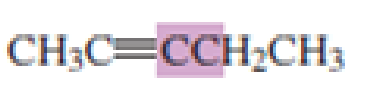
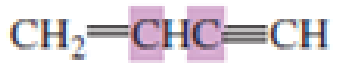
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
CH₂O
and 22
NMR Solvent: CDCl3
IR Solvent: neat
4000
3000
2000
1500
1000
15
[
اند
6,5
9.8
3.0
7.0
6.0
5.0
4.8
3.0
2.0
1.0
9.8
200
100
protons.
Calculate the mass (in grams) of H3AsO4 (MW=141.9416) needed to produce 3.125 x
1026
Please provide with answer, steps and explanation of ideas to solve.
Chapter 6 Solutions
Pearson eText for Essential Organic Chemistry -- Instant Access (Pearson+)
Ch. 6.1 - Draw the mechanism for the reaction of cyclohexene...Ch. 6.2 - a. How many bond orbitals are available for...Ch. 6.2 - Prob. 3PCh. 6.2 - Prob. 4PCh. 6.3 - Prob. 5PCh. 6.3 - Prob. 6PCh. 6.3 - Prob. 7PCh. 6.5 - Prob. 9PCh. 6.5 - Prob. 10PCh. 6.5 - a. What is the major product of each of the...
Ch. 6.5 - Prob. 12PCh. 6.6 - What stereoisomers are obtained from each of the...Ch. 6.6 - Prob. 14PCh. 6.8 - Prob. 15PCh. 6.10 - Name the following:Ch. 6.10 - Draw the structure for each of the following: a....Ch. 6.10 - Draw the structures for and name the seven alkynes...Ch. 6.10 - Name the following:Ch. 6.10 - Name the following:Ch. 6.11 - What hybrid orbitals are used to form the...Ch. 6.13 - Prob. 22PCh. 6.14 - Prob. 23PCh. 6.14 - Which alkyne would be the best one to use for the...Ch. 6.14 - Prob. 25PCh. 6.14 - Prob. 26PCh. 6.15 - Describe the alkyne you would start with and the...Ch. 6.15 - What are products of the following reactions?Ch. 6 - Prob. 29PCh. 6 - Prob. 30PCh. 6 - Prob. 31PCh. 6 - Prob. 32PCh. 6 - What is each compounds systematic name?Ch. 6 - Prob. 34PCh. 6 - Prob. 35PCh. 6 - What reagents could be used to carry out the...Ch. 6 - Prob. 37PCh. 6 - Prob. 38PCh. 6 - Prob. 39PCh. 6 - Prob. 40PCh. 6 - Prob. 41PCh. 6 - Prob. 42PCh. 6 - Answer Problem 42 using 2-butyne as the starting...Ch. 6 - What is each compounds systematic name?Ch. 6 - Prob. 45PCh. 6 - Prob. 46PCh. 6 - Prob. 47PCh. 6 - Prob. 48PCh. 6 - Prob. 49PCh. 6 - Prob. 50PCh. 6 - Draw the keto tautomer for each of the following:Ch. 6 - Propose a mechanism for the following reaction...Ch. 6 - Prob. 53PCh. 6 - Prob. 54PCh. 6 - Prob. 55PCh. 6 - Propose a mechanism for the following reaction:Ch. 6 - Prob. 57PCh. 6 - Prob. 58PCh. 6 - Prob. 59PCh. 6 - Prob. 60PCh. 6 - Prob. 61PCh. 6 - Prob. 62P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please provide with answer, steps and explanation of ideas to solve.arrow_forwardPlease provide with answer, steps and explanation of ideas to solve.arrow_forwardUsing what we have learned in CHEM 2310 and up through class on 1/31, propose a series of reaction steps to achieve the transformation below. Be sure to show all reagents and intermediates for full credit. You do not need to draw mechanism arrows, but you do need to include charges where appropriate. If you do not put your group name, you will get half credit at most. ? Brarrow_forward
- Define metal cluster and cage compound. Give some examples of both.arrow_forwardPlease provide with answer, steps and explanation of ideas to solve.arrow_forwardIndicate whether the copper(II) acetate dimer, in its dihydrated form with the formula [(CH3COO)2Cu]2·2H2O, is a metal cluster, a cage compound, or neither.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License