
Concept explainers
Rank the following ions in order of increasing number of unpaired electrons: Tr3+, Fe2+, V3+, Cu+, Mn4+.
Interpretation: It should be ranked the given set of ions in increasing order of number of unpaired electrons.
Concept Introduction:
- Aufbau Principle tells that the orbital with the lower energy is filled with electrons first and then the filling of higher energy orbital follows. By using this, valence orbital diagram can be draw for any atoms or ions.
- Electronic configuration is the arrangement of the electrons of atoms in the orbital. The electronic configurations are writing for atoms and ions, in accordance with Pauli Exclusion Principle and Hund’s rule.
- According to Pauli Exclusion Principle, no two electrons having the same spin can occupy the same orbital.
- According to Hund’s rule, the orbital in the subshell is filled singly by one electron before the same orbital is doubly filled. When the orbital is singly filled, all the electrons have same spin. In a doubly filled orbital, there are two electrons with opposite spin.
To determine the increasing order of unpaired electrons present in the given set of ions.
Answer to Problem 4.79QP
Ions in increasing order of unpaired electrons,
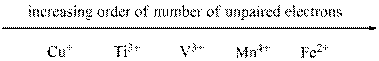
Explanation of Solution
Electronic configuration of
The valence orbital diagram of
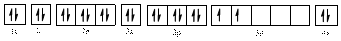
The electronic configuration of
Electronic configuration of
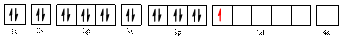
The electronic configuration of
The valence orbital diagram is drawn as shown above. From this, we can find that there is one unpaired electron and which is highlighted in red colour.
Electronic configuration of
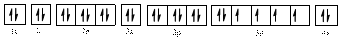
The electronic configuration of
Electronic configuration of
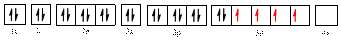
The electronic configuration of
The valence orbital diagram is drawn as shown above. From this, we can find that there are four unpaired electrons that are highlighted in red colour.
Electronic configuration of
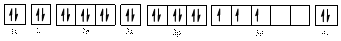
The electronic configuration of
Electronic configuration of
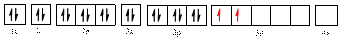
The electronic configuration of
The valence orbital diagram is drawn as shown above. From this, we can find that there are two unpaired electrons, that are highlighted in red colour.
Electronic configuration of
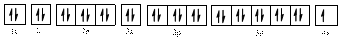
The electronic configuration of
Electronic configuration of
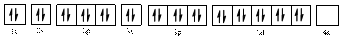
The electronic configuration of
The valence orbital diagram is drawn as shown above. From this we can find that there are no unpaired electrons present.
Electronic configuration of
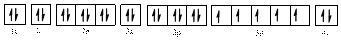
The electronic configuration of
Electronic configuration of
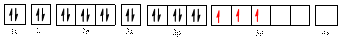
The electronic configuration of
The valence orbital diagram is drawn as shown above. From this, we can find that there are three unpaired electrons present and the same is highlighted in red colour.
Given ions were ranked in increasing order of unpaired electron as follows,
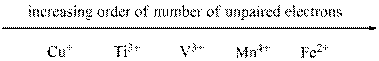
From the number of unpaired electrons for each of the given ions found out in the previous steps we can arrange them in increasing order of number of unpaired electrons as shown above.
The given sets of ions were ranked in increasing order of number of unpaired electrons.
Want to see more full solutions like this?
Chapter 4 Solutions
Chemistry: Atoms First
- Please correct answer and don't used hand raitingarrow_forwardneed help please and thanks dont understand a-b Learning Goal: As discussed during the lecture, the enzyme HIV-1 reverse transcriptae (HIV-RT) plays a significant role for the HIV virus and is an important drug target. Assume a concentration [E] of 2.00 µM (i.e. 2.00 x 10-6 mol/l) for HIV-RT. Two potential drug molecules, D1 and D2, were identified, which form stable complexes with the HIV-RT. The dissociation constant of the complex ED1 formed by HIV-RT and the drug D1 is 1.00 nM (i.e. 1.00 x 10-9). The dissociation constant of the complex ED2 formed by HIV-RT and the drug D2 is 100 nM (i.e. 1.00 x 10-7). Part A - Difference in binding free eenergies Compute the difference in binding free energy (at a physiological temperature T=310 K) for the complexes. Provide the difference as a positive numerical expression with three significant figures in kJ/mol. The margin of error is 2%. Part B - Compare difference in free energy to the thermal energy Divide the…arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardCan you tell me if my answers are correctarrow_forwardBunsenite (NiO) crystallizes like common salt (NaCl), with a lattice parameter a = 4.177 Å. A sample of this mineral that has Schottky defects that are not supposed to decrease the volume of the material has a density of 6.67 g/cm3. What percentage of NiO molecules is missing? (Data: atomic weight of Ni: 58.7; atomic weight of O: 16).arrow_forward
- A sample of aluminum (face-centered cubic - FCC) has a density of 2.695 mg/m3 and a lattice parameter of 4.04958 Å. Calculate the fraction of vacancies in the structure. (Atomic weight of aluminum: 26.981).arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardWhich of the following species is a valid resonance structure of A? Use curved arrows to show how A is converted to any valid resonance structure. When a compound is not a valid resonance structurc of A, explain why not. Provide steps and tips on what to look for to understand how to solve and apply to other problems.arrow_forwardN IZ Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 HN Molecule 3 Х HN www. Molecule 4 Molecule 5 Molecule 6 none of the above NH NH Garrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





