
General, Organic, and Biological Chemistry
7th Edition
ISBN: 9781285853918
Author: H. Stephen Stoker
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 4.61EP
The component elements for four binary ionic compounds are shown with different colors on the following periodic table.
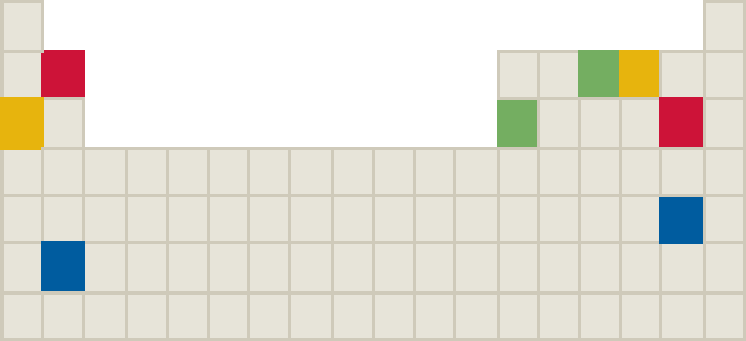
What is the likely chemical formula for the following?
- a. Red compound
- b. Blue compound
- c. Yellow compound
- d. Green compound
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A mixture of 0.568 M H₂O, 0.438 M Cl₂O, and 0.710 M HClO are enclosed in a vessel at 25 °C.
H₂O(g) + C₁₂O(g) = 2 HOCl(g)
K = 0.0900 at 25°C
с
Calculate the equilibrium concentrations of each gas at 25 °C.
[H₂O]=
[C₁₂O]=
[HOCI]=
M
Σ
M
What units (if any) does the response factor (K) have? Does the response factor (K) depend upon how the concentration is expressed (e.g. molarity, ppm, ppb, etc.)?
Provide the structure, circle or draw, of the monomeric unit found in the biological polymeric
materials given below.
HO
OH
amylose
OH
OH
행
3
HO
cellulose
OH
OH
OH
Ho
HO
Chapter 4 Solutions
General, Organic, and Biological Chemistry
Ch. 4.1 - Prob. 1QQCh. 4.1 - Prob. 2QQCh. 4.1 - Prob. 3QQCh. 4.2 - How many valence electrons are present in an atom...Ch. 4.2 - Prob. 2QQCh. 4.2 - Prob. 3QQCh. 4.2 - Prob. 4QQCh. 4.2 - Which of the following elements would have the...Ch. 4.3 - Prob. 1QQCh. 4.3 - Prob. 2QQ
Ch. 4.3 - Prob. 3QQCh. 4.4 - In terms of subatomic particles, a Ca2+ ion...Ch. 4.4 - Prob. 2QQCh. 4.4 - Prob. 3QQCh. 4.4 - Prob. 4QQCh. 4.5 - An atom with a 1s22s22p4 electron configuration...Ch. 4.5 - Prob. 2QQCh. 4.5 - Prob. 3QQCh. 4.5 - Prob. 4QQCh. 4.5 - Prob. 5QQCh. 4.6 - Prob. 1QQCh. 4.6 - Prob. 2QQCh. 4.6 - Prob. 3QQCh. 4.7 - What is the chemical formula of the ionic compound...Ch. 4.7 - What is the chemical formula of the ionic compound...Ch. 4.7 - Given that Z2 ions are present in the ionic...Ch. 4.7 - Prob. 4QQCh. 4.8 - Prob. 1QQCh. 4.8 - Which of the following is a correct description of...Ch. 4.9 - Prob. 1QQCh. 4.9 - Prob. 2QQCh. 4.9 - Prob. 3QQCh. 4.9 - Prob. 4QQCh. 4.9 - Prob. 5QQCh. 4.9 - Prob. 6QQCh. 4.9 - The correct name for the binary ionic compound...Ch. 4.9 - Prob. 8QQCh. 4.10 - Prob. 1QQCh. 4.10 - Which of the following statements about polyatomic...Ch. 4.10 - The nitrate, sulfate, and phosphate ions have,...Ch. 4.10 - Prob. 4QQCh. 4.11 - Prob. 1QQCh. 4.11 - Prob. 2QQCh. 4.11 - Prob. 3QQCh. 4.11 - Prob. 4QQCh. 4.11 - Prob. 5QQCh. 4.11 - What is the chemical formula for the compound...Ch. 4 - Contrast the two general types of chemical bonds...Ch. 4 - Contrast the two general types of chemical...Ch. 4 - How many valence electrons do atoms with the...Ch. 4 - How many valence electrons do atoms with the...Ch. 4 - Prob. 4.5EPCh. 4 - Prob. 4.6EPCh. 4 - Write the complete electron configuration for each...Ch. 4 - Write the complete electron configuration for each...Ch. 4 - Prob. 4.9EPCh. 4 - For each of the following pairs of representative...Ch. 4 - How many of the highlighted elements in the...Ch. 4 - How many of the highlighted elements in the...Ch. 4 - Draw Lewis symbols for atoms of each of the...Ch. 4 - Draw Lewis symbols for atoms of each of the...Ch. 4 - Each of the following Lewis symbols represents a...Ch. 4 - Each of the following Lewis symbols represents a...Ch. 4 - Prob. 4.17EPCh. 4 - Prob. 4.18EPCh. 4 - What is the chemical property of the noble gases...Ch. 4 - Prob. 4.20EPCh. 4 - Prob. 4.21EPCh. 4 - Prob. 4.22EPCh. 4 - Give the chemical symbol for each of the following...Ch. 4 - Give the chemical symbol for each of the following...Ch. 4 - What would be the chemical symbol for an ion with...Ch. 4 - What would be the chemical symbol for an ion with...Ch. 4 - Fill in the blanks in each line in the following...Ch. 4 - Fill in the blanks in each line in the following...Ch. 4 - Fill in the blanks in each line of the following...Ch. 4 - Fill in the blanks in each line of the following...Ch. 4 - Identify element X by giving its chemical symbol,...Ch. 4 - Prob. 4.32EPCh. 4 - Prob. 4.33EPCh. 4 - Prob. 4.34EPCh. 4 - Prob. 4.35EPCh. 4 - Draw Lewis symbols for the following ions. a. O2...Ch. 4 - What is the charge on the monatomic ion formed by...Ch. 4 - What is the charge on the monatomic ion formed by...Ch. 4 - Indicate the number of electrons lost or gained...Ch. 4 - Indicate the number of electrons lost or gained...Ch. 4 - Which noble gas has an electron configuration...Ch. 4 - Prob. 4.42EPCh. 4 - Which noble gas is isoelectronic with each of the...Ch. 4 - Which noble gas is isoelectronic with each of the...Ch. 4 - Prob. 4.45EPCh. 4 - Indicate whether or not each of the following...Ch. 4 - Prob. 4.47EPCh. 4 - Prob. 4.48EPCh. 4 - Prob. 4.49EPCh. 4 - Write the electron configuration of the following....Ch. 4 - How many valence electrons are present in each of...Ch. 4 - Prob. 4.52EPCh. 4 - Using Lewis structures, show how ionic compounds...Ch. 4 - Using Lewis structures, show how ionic compounds...Ch. 4 - The following Lewis symbols for ions have the...Ch. 4 - Prob. 4.56EPCh. 4 - Prob. 4.57EPCh. 4 - Prob. 4.58EPCh. 4 - Prob. 4.59EPCh. 4 - Prob. 4.60EPCh. 4 - The component elements for four binary ionic...Ch. 4 - Prob. 4.62EPCh. 4 - Write the complete chemical formula (symbol and...Ch. 4 - Write the complete chemical formula (symbol and...Ch. 4 - Write the chemical formula for the ionic compound...Ch. 4 - Prob. 4.66EPCh. 4 - Prob. 4.67EPCh. 4 - What is the chemical formula of the ionic compound...Ch. 4 - A representative element (X) forms an ion with a 2...Ch. 4 - A representative element (Z) forms an ion with a...Ch. 4 - Prob. 4.71EPCh. 4 - The following questions pertain to the ionic...Ch. 4 - Prob. 4.73EPCh. 4 - Prob. 4.74EPCh. 4 - Prob. 4.75EPCh. 4 - Prob. 4.76EPCh. 4 - Prob. 4.77EPCh. 4 - Prob. 4.78EPCh. 4 - Prob. 4.79EPCh. 4 - Which of the following binary compounds would be...Ch. 4 - Name the following binary ionic compounds, each of...Ch. 4 - Name the following binary ionic compounds, each of...Ch. 4 - Calculate the charge on the metal ion in the...Ch. 4 - Calculate the charge on the metal ion in the...Ch. 4 - Prob. 4.85EPCh. 4 - Prob. 4.86EPCh. 4 - Prob. 4.87EPCh. 4 - Prob. 4.88EPCh. 4 - Name each of the following binary ionic compounds....Ch. 4 - Name each of the following binary ionic compounds....Ch. 4 - Prob. 4.91EPCh. 4 - Name each compound in the following pairs of...Ch. 4 - Prob. 4.93EPCh. 4 - Write chemical formulas for the following binary...Ch. 4 - Prob. 4.95EPCh. 4 - Write chemical formulas for the following binary...Ch. 4 - Prob. 4.97EPCh. 4 - Prob. 4.98EPCh. 4 - Fill in the blanks in each line of the following...Ch. 4 - Prob. 4.100EPCh. 4 - Prob. 4.101EPCh. 4 - Prob. 4.102EPCh. 4 - Prob. 4.103EPCh. 4 - How many oxygen atoms are present in each of the...Ch. 4 - Prob. 4.105EPCh. 4 - Prob. 4.106EPCh. 4 - Prob. 4.107EPCh. 4 - Prob. 4.108EPCh. 4 - Prob. 4.109EPCh. 4 - Prob. 4.110EPCh. 4 - How many ions are present per formula unit in each...Ch. 4 - Prob. 4.112EPCh. 4 - Name the following compounds, all of which contain...Ch. 4 - Prob. 4.114EPCh. 4 - Prob. 4.115EPCh. 4 - Prob. 4.116EPCh. 4 - Prob. 4.117EPCh. 4 - Write formulas for the following compounds, all of...Ch. 4 - Write chemical formulas for the following...Ch. 4 - Write chemical formulas for the following...Ch. 4 - Prob. 4.121EPCh. 4 - Prob. 4.122EPCh. 4 - Prob. 4.123EPCh. 4 - Prob. 4.124EP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- OA. For the structure shown, rank the bond lengths (labeled a, b and c) from shortest to longest. Place your answer in the box. Only the answer in the box will be graded. (2 points) H -CH3 THe b Нarrow_forwardDon't used hand raitingarrow_forwardQuizzes - Gen Organic & Biological Che... ☆ myd21.lcc.edu + O G screenshot on mac - Google Search savings hulu youtube google disney+ HBO zlib Homework Hel...s | bartleby cell bio book Yuzu Reader: Chemistry G periodic table - Google Search b Home | bartleby 0:33:26 remaining CHEM 120 Chapter 5_Quiz 3 Page 1: 1 > 2 > 3 > 6 ¦ 5 > 4 > 7 ¦ 1 1 10 8 ¦ 9 a ¦ -- Quiz Information silicon-27 A doctor gives a patient 0.01 mC i of beta radiation. How many beta particles would the patient receive in I minute? (1 Ci = 3.7 x 10 10 d/s) Question 5 (1 point) Saved Listen 2.22 x 107 222 x 108 3.7 x 108 2.22 x 108 none of the above Question 6 (1 point) Listen The recommended dosage of 1-131 for a test is 4.2 μCi per kg of body mass. How many millicuries should be given to a 55 kg patient? (1 mCi = 1000 μСi)? 230 mCiarrow_forward
- Q4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution, respectively. F CI Br | Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to have a reasonable yield of product. NH2 Br Br Br .OH Brarrow_forwardClassify each molecule as optically active or inactive. Determine the configuration at each H соон Chirality center OH 애 He OH H3C Ноос H H COOH A K B.arrow_forwardQ1: Rank the relative nucleophilicity of the following species in ethanol. CH3O¯, CH3OH, CH3COO, CH3COOH, CH3S Q2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY