
Concept explainers
Fill in the blanks in each line of the following table. The first line is already completed as an example.
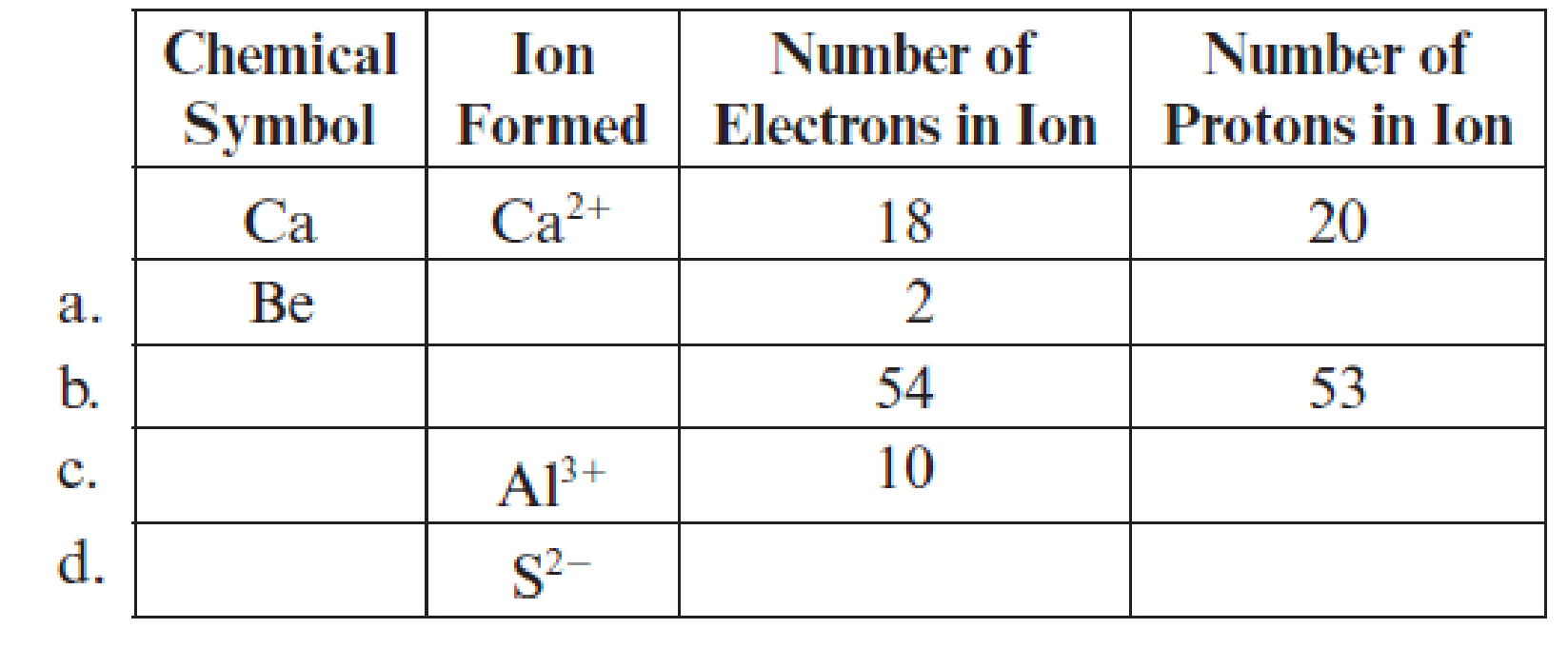
(a)
Interpretation:
Filling of each blank in the following table has to be done:
Concept Introduction:
Atoms are composed of three types of particles called subatomic particles. They are as follows:
- Protons: Positively charged particles in an atom.
- Neutrons: Neutral charged particles in an atom.
- Electrons: Negatively charged particles in an atom.
The neutral atom has equal number of protons and electrons. Gaining or loosing of electrons of an atom forms ion.
Negative charged ions are formed by gaining one or more electrons and it has more electrons than protons.
Positive charged ions are formed by losing one or more electrons and it has more protons than electrons.
Answer to Problem 4.29EP
Complete table is shown below:
Explanation of Solution
The chemical symbol of the element is
Two electrons are lesser than the total number protons thatmeans it lost two electrons and the charge of the beryllium ion is
Hence, the symbol of ion is
(b)
Interpretation:
Filling of each blank in the following table has to be done:
Concept Introduction:
Atoms are composed of three types of particles called subatomic particles. They are as follows:
- Protons: Positively charged particles in an atom.
- Neutrons: Neutral charged particles in an atom.
- Electrons: Negatively charged particles in an atom.
The neutral atom has equal number of protons and electrons. Gaining or loosing of electrons of an atom form ion.
Negative charged ions are formed by gaining one or more electrons and it has more electrons than protons.
Positive charged ions are formed by losing one or more electrons and it has more protons than electrons.
Answer to Problem 4.29EP
Complete table is shown below:
Explanation of Solution
The element has
Hence, the number of protons are
(c)
Interpretation:
Filling of each blank in the following table has to be done:
Concept Introduction:
Atoms are composed of three types of particles called subatomic particles. They are as follows:
- Protons: Positively charged particles in an atom.
- Neutrons: Neutral charged particles in an atom.
- Electrons: Negatively charged particles in an atom.
The neutral atom has equal number of protons and electrons. Gaining or loosing of electrons of an atom forms ion.
Negative charged ions are formed by gaining one or more electrons and it has more electrons than protons.
Positive charged ions are formed by losing one or more electrons and it has more protons than electrons.
Answer to Problem 4.29EP
Complete table is shown below:
Explanation of Solution
The ion
Hence, ion
(d)
Interpretation:
Filling of each blank in the following table has to be done:
Concept Introduction:
Atoms are composed of three types of particles called subatomic particles. They are as follows:
- Protons: Positively charged particles in an atom.
- Neutrons: Neutral charged particles in an atom.
- Electrons: Negatively charged particles in an atom.
The neutral atom has equal number of protons and electrons. Gaining or loosing of electrons of an atom forms ion.
Negative charged ions are formed by gaining one or more electrons and it has more electrons than protons.
Positive charged ions are formed by losing one or more electrons and it has more protons than electrons.
Answer to Problem 4.29EP
Complete table is shown below:
Explanation of Solution
The given ion is
The charge of sulfur atom is
Hence, ion is
Want to see more full solutions like this?
Chapter 4 Solutions
General, Organic, and Biological Chemistry
- Please draw by handarrow_forward3. Predict the major product and give a mechanism for the following reactions: (CH3)3COH/H₂SO4 a) b) NC CH₂O c) LOCH, (CH3)3COH/H2SO4 H,SO -OHarrow_forwardIndicate if the aldehyde shown reacts with the provided nucleophiles in acid or base conditions. a NaBH4 be Li eli -NH2 P(Ph3) f KCN g OH excess h CH3OH i NaCHCCH3arrow_forward
- Predict the major products of the following organic reaction: + A ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forwardPolar solutes are most likely to dissolve into _____, and _____ are most likely to dissolve into nonpolar solvents. A. nonpolar solutes; polar solvents B. nonpolar solvents; polar solvents C. polar solvents; nonpolar solutes D. polar solutes; nonpolar solventsarrow_forwardDeducing the Peactants Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Xarrow_forward
- Draw all 8 stereoisomers, circling each pair of enantiomer(s)/ mirror image compound(s)arrow_forwardBookmarks Profiles Tab Window Help Chemical Formula - Aktiv Che X + → C 11 a app.aktiv.com Google Chrome isn't your default browser Set as default Question 12 of 16 Q Fri Feb 2 Verify it's you New Chrome availabl- Write the balanced molecular chemical equation for the reaction in aqueous solution for mercury(I) nitrate and chromium(VI) sulfate. If no reaction occurs, simply write only NR. Be sure to include the proper phases for all species within the reaction. 3 Hg(NO3)2(aq) + Cг2(SO4)3(aq) → 3 Hg₂SO (s) + 2 Cr(NO3), (aq) ean Ui mate co ence an climate bility inc ulnerabili women, main critic CLIMATE-INI ernational + 10 O 2 W FEB 1 + 4- 3- 2- 2 2 ( 3 4 NS 28 2 ty 56 + 2+ 3+ 4+ 7 8 9 0 5 (s) (1) Ch O 8 9 (g) (aq) Hg NR CI Cr x H₂O A 80 Q A DII A F2 F3 FA F5 F6 F7 F8 F9 #3 EA $ do 50 % 6 CO & 7 E R T Y U 8 ( 9 0 F10 34 F11 川 F12 Subr + delete 0 { P }arrow_forwardDeducing the reactants of a Diels-Alder reaction n the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. >arrow_forward
- Predict the major products of the following organic reaction: + Some important notes: A ? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure.arrow_forwardif the answer is no reaction than state that and please hand draw!arrow_forward"I have written solutions in text form, but I need experts to rewrite them in handwriting from A to Z, exactly as I have written, without any changes."arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





