
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 5E
Consider the following orbital representation of HCCH (ethyne).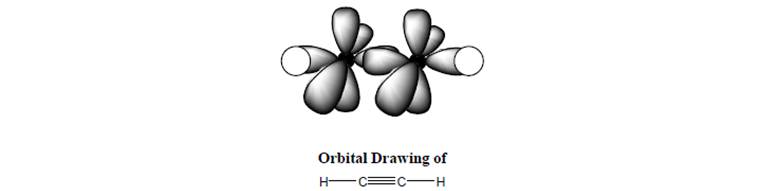
a. Answer the same three questions (a-c) from the previous exercise.
b. Label each
c. What is the total number of a bonds found in ethyne?….
d. How many p orbitals are there on a single carbon of ethyne?
e. How many hybrid orbitals are there on a single carbon of ethyne?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Predict the product formed when the compound shown below undergoes a reaction with MCPBA in CH2Cl2. MCPBA is meta-chloroperoxybenzoic acid.
k
https://app.aktiv.com
STARTING AMOUNT
6 58°F
Clear
+
F1
X
Dimensional Analysis - Aktiv Chemistry
Your Aktiv Learning trial expires on 02/25/25 at 02:14 PM
Question 19 of 22
Polyethylene terephthalate (PET) is used in plastic water bottles. A water bottle has a
mass of 14.0 grams. Given a density of 1.38 g/cm³, what is the volume of the
plastic used to make the water bottle in cm³ ?
ADD FACTOR
ANSWER
RESET
ว
100
14.0
0.01
10.1
1000
0.099
1.38
0.001
Q Search
F5
-O+
F6
F7
+
F3
F2
W
E
S4
ST
#3
F4
%
5
Y
R
S
&
7
cm³
g/cm³
g
ם
F8
* 00
8
F9
P
ل
DOD
S
F10
F11
F12
Insert
D
F
G
H
J
K
+ 11
A doctor gives a patient 10 Ci of beta radiation. How many betaparticles would the patient receive in 1 minute? (1 Ci = 3.7 x 1010d/s)
Chapter 3 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 3 - Prob. 1CTQCh. 3 - What neutral atom is represented by the electron...Ch. 3 - Prob. 3CTQCh. 3 - Consider any one of the four identical hybrid...Ch. 3 - Prob. 5CTQCh. 3 - Prob. 6CTQCh. 3 - Prob. 7CTQCh. 3 - Prob. 8CTQCh. 3 - Prob. 9CTQCh. 3 - Prob. 10CTQ
Ch. 3 - On the left side of Figure 3.6, label the areas...Ch. 3 - Prob. 12CTQCh. 3 - Prob. 13CTQCh. 3 - Prob. 14CTQCh. 3 - Prob. 15CTQCh. 3 - Now consider the fully formed molecule on the...Ch. 3 - Prob. 1ECh. 3 - Explain why the two molecules below cannot...Ch. 3 - Prob. 3ECh. 3 - Consider the incomplete orbital representation of...Ch. 3 - Consider the following orbital representation of...Ch. 3 - Summarize how one determines the hybridization...Ch. 3 - Explain what is wrong with each of the following...Ch. 3 - Prob. 8ECh. 3 - Prob. 9ECh. 3 - Complete the following tables, and memorize their...Ch. 3 - Draw orbital representations of bonding in water...Ch. 3 - Draw electron configuration diagrams for carbon in...Ch. 3 - Prob. 13E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Part C IN H N. Br₂ (2 equiv.) AlBr3 Draw the molecule on the canvas by choosing buttons from the Tools (for bonds and + e (×) H± 12D T EXP. L CONT. דarrow_forward9. OA. Rank the expected boiling points of the compounds shown below from highest to lowest. Place your answer appropriately in the box. Only the answer in the box will be graded. (3) points) OH OH بر بد بدید 2 3arrow_forwardThere is an instrument in Johnson 334 that measures total-reflectance x-ray fluorescence (TXRF) to do elemental analysis (i.e., determine what elements are present in a sample). A researcher is preparing a to measure calcium content in a series of well water samples by TXRF with an internal standard of vanadium (atomic symbol: V). She has prepared a series of standard solutions to ensure a linear instrument response over the expected Ca concentration range of 40-80 ppm. The concentrations of Ca and V (ppm) and the instrument response (peak area, arbitrary units) are shown below. Also included is a sample spectrum. Equation 1 describes the response factor, K, relating the analyte signal (SA) and the standard signal (SIS) to their respective concentrations (CA and CIS). Ca, ppm V, ppm SCa, arb. units SV, arb. units 20.0 10.0 14375.11 14261.02 40.0 10.0 36182.15 17997.10 60.0 10.0 39275.74 12988.01 80.0 10.0 57530.75 14268.54 100.0…arrow_forward
- A mixture of 0.568 M H₂O, 0.438 M Cl₂O, and 0.710 M HClO are enclosed in a vessel at 25 °C. H₂O(g) + C₁₂O(g) = 2 HOCl(g) K = 0.0900 at 25°C с Calculate the equilibrium concentrations of each gas at 25 °C. [H₂O]= [C₁₂O]= [HOCI]= M Σ Marrow_forwardWhat units (if any) does the response factor (K) have? Does the response factor (K) depend upon how the concentration is expressed (e.g. molarity, ppm, ppb, etc.)?arrow_forwardProvide the structure, circle or draw, of the monomeric unit found in the biological polymeric materials given below. HO OH amylose OH OH 행 3 HO cellulose OH OH OH Ho HOarrow_forward
- OA. For the structure shown, rank the bond lengths (labeled a, b and c) from shortest to longest. Place your answer in the box. Only the answer in the box will be graded. (2 points) H -CH3 THe b Нarrow_forwardDon't used hand raitingarrow_forwardQuizzes - Gen Organic & Biological Che... ☆ myd21.lcc.edu + O G screenshot on mac - Google Search savings hulu youtube google disney+ HBO zlib Homework Hel...s | bartleby cell bio book Yuzu Reader: Chemistry G periodic table - Google Search b Home | bartleby 0:33:26 remaining CHEM 120 Chapter 5_Quiz 3 Page 1: 1 > 2 > 3 > 6 ¦ 5 > 4 > 7 ¦ 1 1 10 8 ¦ 9 a ¦ -- Quiz Information silicon-27 A doctor gives a patient 0.01 mC i of beta radiation. How many beta particles would the patient receive in I minute? (1 Ci = 3.7 x 10 10 d/s) Question 5 (1 point) Saved Listen 2.22 x 107 222 x 108 3.7 x 108 2.22 x 108 none of the above Question 6 (1 point) Listen The recommended dosage of 1-131 for a test is 4.2 μCi per kg of body mass. How many millicuries should be given to a 55 kg patient? (1 mCi = 1000 μСi)? 230 mCiarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY