
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 29.SE, Problem 31MP
Interpretation Introduction
Interpretation:
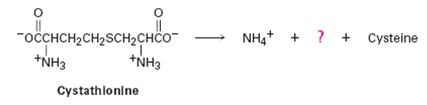
The amino acid cysteine, C3H7NO2S, is biosynthesized from a substance called cystathionine by a multistep pathway.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the stepwise mechanism for this reaction?
Draw the major product of this reaction
Please provide the IUPAC name for the compound shown here
Chapter 29 Solutions
Organic Chemistry
Ch. 29.1 - Prob. 1PCh. 29.3 - Write the equations for the remaining passages of...Ch. 29.3 - Prob. 3PCh. 29.4 - Write a mechanism for the dehydration reaction of...Ch. 29.4 - Evidence for the role of acetate in fatty-acid...Ch. 29.4 - Does the reduction of acetoacetyl ACP in step 6...Ch. 29.5 - Prob. 7PCh. 29.5 - Look at the entire glycolysis pathway, and make a...Ch. 29.6 - Prob. 9PCh. 29.7 - Prob. 10P
Ch. 29.7 - Write mechanisms for step 2 of the citric acid...Ch. 29.7 - Prob. 12PCh. 29.8 - Prob. 13PCh. 29.9 - Write all the steps in the transamination reaction...Ch. 29.9 - What -keto acid is formed on transamination of...Ch. 29.9 - Prob. 16PCh. 29.SE - Prob. 17VCCh. 29.SE - Identify the following intermediate in the citric...Ch. 29.SE - The following compound is an intermediate in the...Ch. 29.SE - Prob. 20VCCh. 29.SE - In the pentose phosphate pathway for degrading...Ch. 29.SE - Prob. 22MPCh. 29.SE - One of the steps in the pentose phosphate pathway...Ch. 29.SE - One of the steps in the pentose phosphate pathway...Ch. 29.SE - Prob. 25MPCh. 29.SE - Prob. 26MPCh. 29.SE - Prob. 27MPCh. 29.SE - Prob. 28MPCh. 29.SE - Prob. 29MPCh. 29.SE - Prob. 30MPCh. 29.SE - Prob. 31MPCh. 29.SE - Prob. 32APCh. 29.SE - Prob. 33APCh. 29.SE - Prob. 34APCh. 29.SE - Prob. 35APCh. 29.SE - Prob. 36APCh. 29.SE - Prob. 37APCh. 29.SE - Prob. 38APCh. 29.SE - Prob. 39APCh. 29.SE - Prob. 40APCh. 29.SE - Prob. 41APCh. 29.SE - Prob. 42APCh. 29.SE - Prob. 43APCh. 29.SE - Prob. 44APCh. 29.SE - Prob. 45APCh. 29.SE - Prob. 46APCh. 29.SE - Prob. 47APCh. 29.SE - Prob. 48APCh. 29.SE - Prob. 49APCh. 29.SE - Prob. 50APCh. 29.SE - In glycerol metabolism, the oxidation of...Ch. 29.SE - Prob. 52APCh. 29.SE - Prob. 53APCh. 29.SE - Prob. 54APCh. 29.SE - In step 7 of fatty-acid biosynthesis (Figure...Ch. 29.SE - Prob. 56AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Problem 6-29 Identify the functional groups in the following molecules, and show the polarity of each: (a) CH3CH2C=N CH, CH, COCH (c) CH3CCH2COCH3 NH2 (e) OCH3 (b) (d) O Problem 6-30 Identify the following reactions as additions, eliminations, substitutions, or rearrangements: (a) CH3CH2Br + NaCN CH3CH2CN ( + NaBr) Acid -OH (+ H2O) catalyst (b) + (c) Heat NO2 Light + 02N-NO2 (+ HNO2) (d)arrow_forwardPredict the organic product of Y that is formed in the reaction below, and draw the skeletal ("line") structures of the missing organic product. Please include all steps & drawings & explanations.arrow_forwardPlease choose the best reagents to complete the following reactionarrow_forward
- Problem 6-17 Look at the following energy diagram: Energy Reaction progress (a) Is AG for the reaction positive or negative? Label it on the diagram. (b) How many steps are involved in the reaction? (c) How many transition states are there? Label them on the diagram. Problem 6-19 What is the difference between a transition state and an intermediate? Problem 6-21 Draw an energy diagram for a two-step reaction with Keq > 1. Label the overall AG°, transition states, and intermediate. Is AG° positive or negative? Problem 6-23 Draw an energy diagram for a reaction with Keq = 1. What is the value of AG° in this reaction?arrow_forwardProblem 6-37 Draw the different monochlorinated constitutional isomers you would obtain by the radical chlorination of the following compounds. (b) (c) Problem 6-39 Show the structure of the carbocation that would result when each of the following alkenes reacts with an acid, H+. (a) (b) (c)arrow_forwardPlease draw the major product of this reaction. Ignore inorganic byproducts and the carboxylic side productarrow_forward
- predict the product formed by the reaction of one mole each of cyclohex-2-en-1-one and lithium diethylcuprate. Assume a hydrolysis step follows the additionarrow_forwardPlease handwriting for questions 1 and 3arrow_forwardIs (CH3)3NHBr an acidic or basic salt? What happens when dissolved in aqueous solution? Doesn't it lose a Br-? Does it interact with the water? Please advise.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY