
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 29.4, Problem 6P
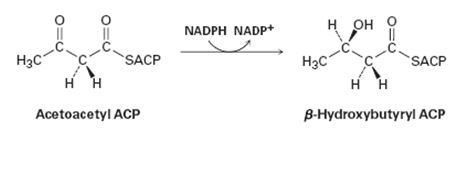
Does the reduction of acetoacetyl ACP in step 6 occur on the Re face or the Si face of the molecule?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Calculating the pH of a weak base titrated with a strong acid
An analytical chemist is titrating 184.2 mL of a 0.7800M solution of dimethylamine ((CH3)
NH with a 0.3000M solution of HClO4. The pK₁ of
dimethylamine is 3.27. Calculate the pH of the base solution after the chemist has added 424.1 mL of the HClO solution to it.
2
4
Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO 4 solution added.
Round your answer to 2 decimal places.
pH = ☐
☑
?
000
18
Ar
1
B
Using the Nernst equation to calculate nonstandard cell voltage
A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction:
MnO2 (s)+4H* (aq)+2Cr²+ (aq) → Mn²+ (aq)+2H₂O (1)+2Cr³+ (aq)
+
2+
2+
3+
Suppose the cell is prepared with 7.44 M H* and 0.485 M Cr²+ in one half-cell and 7.92 M Mn² and 3.73 M Cr³+ in the other.
Calculate the cell voltage under these conditions. Round your answer to 3 significant digits.
☐
x10
μ
Х
5
?
000
日。
Calculating standard reaction free energy from standard reduction...
Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction.
Be sure your answer has the correct number of significant digits.
NO (g) +H₂O (1) + Cu²+ (aq) → HNO₂ (aq) +H* (aq)+Cu* (aq)
kJ
-
☐ x10
x10
olo
18
Ar
Chapter 29 Solutions
Organic Chemistry
Ch. 29.1 - Prob. 1PCh. 29.3 - Write the equations for the remaining passages of...Ch. 29.3 - Prob. 3PCh. 29.4 - Write a mechanism for the dehydration reaction of...Ch. 29.4 - Evidence for the role of acetate in fatty-acid...Ch. 29.4 - Does the reduction of acetoacetyl ACP in step 6...Ch. 29.5 - Prob. 7PCh. 29.5 - Look at the entire glycolysis pathway, and make a...Ch. 29.6 - Prob. 9PCh. 29.7 - Prob. 10P
Ch. 29.7 - Write mechanisms for step 2 of the citric acid...Ch. 29.7 - Prob. 12PCh. 29.8 - Prob. 13PCh. 29.9 - Write all the steps in the transamination reaction...Ch. 29.9 - What -keto acid is formed on transamination of...Ch. 29.9 - Prob. 16PCh. 29.SE - Prob. 17VCCh. 29.SE - Identify the following intermediate in the citric...Ch. 29.SE - The following compound is an intermediate in the...Ch. 29.SE - Prob. 20VCCh. 29.SE - In the pentose phosphate pathway for degrading...Ch. 29.SE - Prob. 22MPCh. 29.SE - One of the steps in the pentose phosphate pathway...Ch. 29.SE - One of the steps in the pentose phosphate pathway...Ch. 29.SE - Prob. 25MPCh. 29.SE - Prob. 26MPCh. 29.SE - Prob. 27MPCh. 29.SE - Prob. 28MPCh. 29.SE - Prob. 29MPCh. 29.SE - Prob. 30MPCh. 29.SE - Prob. 31MPCh. 29.SE - Prob. 32APCh. 29.SE - Prob. 33APCh. 29.SE - Prob. 34APCh. 29.SE - Prob. 35APCh. 29.SE - Prob. 36APCh. 29.SE - Prob. 37APCh. 29.SE - Prob. 38APCh. 29.SE - Prob. 39APCh. 29.SE - Prob. 40APCh. 29.SE - Prob. 41APCh. 29.SE - Prob. 42APCh. 29.SE - Prob. 43APCh. 29.SE - Prob. 44APCh. 29.SE - Prob. 45APCh. 29.SE - Prob. 46APCh. 29.SE - Prob. 47APCh. 29.SE - Prob. 48APCh. 29.SE - Prob. 49APCh. 29.SE - Prob. 50APCh. 29.SE - In glycerol metabolism, the oxidation of...Ch. 29.SE - Prob. 52APCh. 29.SE - Prob. 53APCh. 29.SE - Prob. 54APCh. 29.SE - In step 7 of fatty-acid biosynthesis (Figure...Ch. 29.SE - Prob. 56AP
Additional Science Textbook Solutions
Find more solutions based on key concepts
To test your knowledge, discuss the following topics with a study partner or in writing ideally from memory. Th...
HUMAN ANATOMY
Why are mutants used as test organisms in the Ames test?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculating the pH of a weak base titrated with a strong acid b An analytical chemist is titrating 116.9 mL of a 0.7700M solution of aniline (C6H5NH2) with a 0.5300M solution of HNO3. The pK of aniline is 9.37. Calculate the pH of the base solution after the chemist has added 184.2 mL of the HNO 3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☐ ☑ 5arrow_forwardQUESTION: Find the standard deviation for the 4 different groups 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5.033 4.044 334.6 268.7 4.706 3.621 305.6 234.4 4.816 3.728 340.0 262.7 4.828 4.496 304.3 283.2 4.993 3.865 244.7 143.6 STDEV = STDEV = STDEV = STDEV =arrow_forwardQUESTION: Fill in the answers in the empty green boxes regarding 'Question 5: Calculating standard error of regression' *The images of the data showing 'coefficients for the standard curve' have been providedarrow_forward
- Using the Nernst equation to calculate nonstandard cell voltage Try Again Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 2+ 2+ Sn²+ Ba(s) (aq) + Ba (s) Sn (s) + Ba²+ (aq) →>> Suppose the cell is prepared with 6.10 M Sn 2+ 2+ in one half-cell and 6.62 M Ba in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. 1.71 V ☐ x10 ☑ 5 0/5 ? 00. 18 Ararrow_forwardQuestion: Find both the b (gradient) and a (y-intercept) value from the list of data below: (x1 -x̄) 370.5 (y1 - ȳ) 5.240 (x2 - x̄) 142.5 (y2 - ȳ) 2.004 (x3 - x̄) 28.5 (y3 - ȳ) 0.390 (x4 - x̄) -85.5 (y4 - ȳ) -1.231 (x5 - x̄) -199.5 (y5 - ȳ) -2.829 (x6 - x̄) -256.5 (y6 - ȳ) -3.575arrow_forwardCalculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3Cu+ (aq) + Cro²¯ (aq) +4H₂O (1) → 3Cu²+ (aq) +Cr(OH)3 (s)+5OH˜¯ (aq) 0 kJ ☐ x10 00. 18 Ararrow_forward
- Calculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 241.7 mL of a 0.4900M solution of methylamine (CH3NH2) with a 0.7800M solution of HNO3. The pK of methylamine is 3.36. Calculate the pH of the base solution after the chemist has added 17.7 mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☑ ? 18 Ararrow_forwardThe following is two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0mmol/L 262.7mmol/L QUESTION: For both groups (Regular & Salt Reduced tomato sauce) of data provide answers to the following calculations below: 1. Standard Deviation (Sx) 2. T Values (t0.05,4) 3. 95% Confidence Interval (mmol/L) 4. [Na+] (mg/100 mL) 5. 95% Confidence Interval (mg/100 mL)arrow_forward
- If we have leucine (2-amino-4-methylpentanoic acid), alanine (2-aminopropanoic acid) and phenylalanine (2-amino-3-phenylpropanoic acid), indicate the tripeptides that can be formed (use the abbreviated symbols Leu., Ala and Phe).arrow_forwardBriefly state why trifluoroacetic acid is more acidic than acetic acid.arrow_forwardExplain why acid chlorides are more reactive than amides in reactions with nucleophiles.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License