
Concept explainers
a)
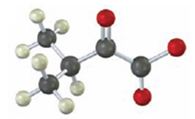
Interpretation:
To identify the amino acid that is a catabolic precursor of the given α-keto acids:
Answer to Problem 17VC
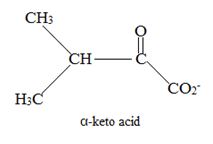
Explanation of Solution
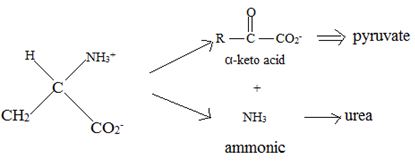
Catabolism of proteins occurs by a discrimination of α – amino acids according to the following reaction:
The retro symmetric strategy is used to derive synthons and starting materials.
b)
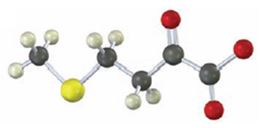
Interpretation:
To identify the amino acid that is a catabolic precursor of the given α-keto acids:
Answer to Problem 17VC
The corresponding α amino acid precursor is respectively:
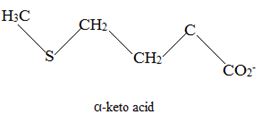
Explanation of Solution
Catabolism of proteins occurs by a discrimination of α – amino acids according to the following reaction:
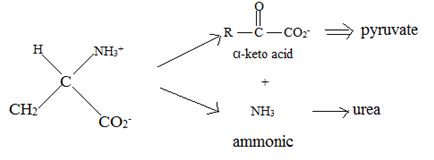
Thus, the α – keto acid derives its structure from its precursor parent amino acid by replacing the 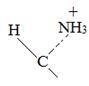 segment by
segment by 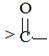 .
.
This proves the above assigned structures for the α – amino acids.
The retro symmetric strategy is used to derive synthons and starting materials.
Want to see more full solutions like this?
Chapter 29 Solutions
Organic Chemistry
- Consider the reaction of the cyclopentanone derivative shown below. i) NaOCH2CH3 CH3CH2OH, 25°C ii) CH3!arrow_forwardWhat constitutes a 'reference material', and why does its utilization play a critical role in the chemical analysis of food products? Provide examples.arrow_forwardExplain what calibration is and why it is essential in relation to food analysis. Provide examples.arrow_forward
- The cobalt mu-hydroxide complex cobaltate(III) of potassium is a dinuclear complex. Correct?arrow_forwardThe cobalt mi-hydroxide complex cobaltate(III) of potassium is a dinuclear complex. Correct?arrow_forward3. Arrange the different acids in Exercise B # 2 from the strongest (1) to the weakest acid (10). 1. 2. (strongest) 3. 4. 5. 6. 7. 8. 9. 10 10. (weakest)arrow_forward
- Name Section Score Date EXERCISE B pH, pOH, pка, AND PKD CALCULATIONS 1. Complete the following table. Solution [H+] [OH-] PH РОН Nature of Solution A 2 x 10-8 M B 1 x 10-7 M C D 12.3 6.8 2. The following table contains the names, formulas, ka or pka for some common acids. Fill in the blanks in the table. (17 Points) Acid Name Formula Dissociation reaction Ka pka Phosphoric acid H₂PO₁ H3PO4 H++ H₂PO 7.08 x 10-3 Dihydrogen H₂PO H₂PO H+ HPO 6.31 x 10-6 phosphate Hydrogen HPO₁ 12.4 phosphate Carbonic acid H2CO3 Hydrogen HCO 6.35 10.3 carbonate or bicarbonate Acetic acid CH,COOH 4.76 Lactic acid CH₂CHOH- COOH 1.38 x 10 Ammonium NH 5.63 x 10-10 Phenol CH₂OH 1 x 10-10 Protonated form CH3NH3* 3.16 x 10-11 of methylaminearrow_forwardIndicate whether it is true that Co(III) complexes are very stable.arrow_forwardMnO2 acts as an oxidant in the chlorine synthesis reaction.arrow_forward
- In Potassium mu-dihydroxydicobaltate (III) tetraoxalate K4[Co2(C2O4)4(OH)2], indicate whether the OH ligand type is bidentate.arrow_forwardImagine an electrochemical cell based on these two half reactions with electrolyte concentrations as given below: Oxidation: Pb(s) → Pb2+(aq, 0.10 M) + 2 e– Reduction: MnO4–(aq, 1.50 M) + 4 H+(aq, 2.0 M) + 3 e– → MnO2(s) + 2 H2O(l) Calculate Ecell (assuming temperature is standard 25 °C).arrow_forward: ☐ + Draw the Fischer projection of the most common naturally-occurring form of aspartate, with the acid group at the top and the side chain at the bottom. Important: be sure your structure shows the molecule as it would exist at physiological pH. Click and drag to start drawing a structure. ✓arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning






