
EP GENERAL CHEMISTRY-MOD.MASTERINGCHEM.
11th Edition
ISBN: 9780133897340
Author: Petrucci
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 24, Problem 65FP
Interpretation Introduction
Interpretation:
The oxidation states of Co ions in the following compound and the number of unpaired electrons (if the complex is low spin) should be determined also the structures of the two optical isomers should be drawn.
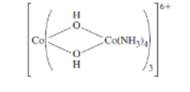
Concept introduction:
A special class of compounds is complex compounds in which the central atom(s)/ion(s) are bonded to the number of neutral or anion molecules. These compounds are also said to be a complex compound.
The bonded number of neutral or anion molecules to central metal is known as ligands that formally donate electrons to the metal center.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the Fischer projection for a D-aldo-pentose. (aldehyde pentose). How many total
stereoisomers are there? Name the sugar you drew.
Draw the Fischer projection for a L-keto-hexose. (ketone pentose). How many total
stereoisomers are there? Draw the enantiomer.
Draw a structure using wedges and dashes for the following compound:
H-
Et
OH
HO-
H
H-
Me
OH
Which of the following molecules are NOT typical carbohydrates? For the molecules that are
carbohydrates, label them as an aldose or ketose.
HO
Он
ОН ОН
Он
ОН
но
ΤΗ
HO
ОН
HO
eve
Он он
ОН
ОН
ОН
If polyethylene has an average molecular weight of 25,000 g/mol, how many repeat units
are present?
Chapter 24 Solutions
EP GENERAL CHEMISTRY-MOD.MASTERINGCHEM.
Ch. 24 - Prob. 1ECh. 24 - Prob. 2ECh. 24 - Prob. 3ECh. 24 - Write appropriate formulas for the following. a....Ch. 24 - Prob. 5ECh. 24 - Prob. 6ECh. 24 - Prob. 7ECh. 24 - Prob. 8ECh. 24 - Prob. 9ECh. 24 - Prob. 10E
Ch. 24 - Prob. 11ECh. 24 - Prob. 12ECh. 24 - If A, B, C, and D are four different ligands, a....Ch. 24 - Prob. 14ECh. 24 - Prob. 15ECh. 24 - The structures of four complex ions are given....Ch. 24 - Prob. 17ECh. 24 - Prob. 18ECh. 24 - Prob. 19ECh. 24 - In contrast to the case of Co2+ considered in...Ch. 24 - Prob. 21ECh. 24 - Prob. 22ECh. 24 - Prob. 23ECh. 24 - Prob. 24ECh. 24 - Prob. 25ECh. 24 - Prob. 26ECh. 24 - Prob. 27ECh. 24 - Prob. 28ECh. 24 - Prob. 29ECh. 24 - Prob. 30ECh. 24 - Prob. 31ECh. 24 - Prob. 32ECh. 24 - Prob. 33ECh. 24 - Prob. 34ECh. 24 - Prob. 35ECh. 24 - Prob. 36ECh. 24 - Prob. 37ECh. 24 - Draw dashed and solid wedge diagrams of...Ch. 24 - Prob. 39IAECh. 24 - Prob. 40IAECh. 24 - Prob. 41IAECh. 24 - Prob. 42IAECh. 24 - Prob. 43IAECh. 24 - Prob. 44IAECh. 24 - Prob. 45IAECh. 24 - Prob. 46IAECh. 24 - Prob. 47IAECh. 24 - Prob. 48IAECh. 24 - Prob. 49IAECh. 24 - Prob. 50IAECh. 24 - Prob. 51IAECh. 24 - Prob. 52IAECh. 24 - Prob. 53IAECh. 24 - Prob. 54IAECh. 24 - The compound CoCl22H2O4NH2 may be one of the...Ch. 24 - Prob. 56IAECh. 24 - Provide a valence bond description of the bonding...Ch. 24 - Prob. 58IAECh. 24 - Prob. 59IAECh. 24 - Prob. 60IAECh. 24 - Prob. 61IAECh. 24 - Prob. 62IAECh. 24 - The graph that follows represents the molar...Ch. 24 - Prob. 64FPCh. 24 - Prob. 65FPCh. 24 - The crystal field stabilization energy (CFSE) can...Ch. 24 - In your own words, describe the following terms or...Ch. 24 - Briefly describe each of the following ideas,...Ch. 24 - Prob. 69SAECh. 24 - The oxidation state of Ni in the complex ion...Ch. 24 - Prob. 71SAECh. 24 - Prob. 72SAECh. 24 - Prob. 73SAECh. 24 - Prob. 74SAECh. 24 - Prob. 75SAECh. 24 - The most soluble of the following solids in...Ch. 24 - Prob. 77SAECh. 24 - Write appropriate formulas for the following...Ch. 24 - Prob. 79SAECh. 24 - Prob. 80SAECh. 24 - Prob. 81SAECh. 24 - Prob. 82SAECh. 24 - Prob. 83SAECh. 24 - Prob. 84SAE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the a-anomer cyclized pyranose Haworth projection of the below hexose. Circle the anomeric carbons. Number the carbons on the Fischer and Haworth projections. Assign R and S for each chiral center. HO CHO -H HO -H H- -OH H -OH CH₂OH Draw the ẞ-anomer cyclized furanose Haworth projection for the below hexose. Circle the anomeric carbons. Number the carbons on the Fischer and Haworth projections. HO CHO -H H -OH HO -H H -OH CH₂OHarrow_forwardName the below disaccharide. Circle any hemiacetals. Identify the numbering of glycosidic linkage, and identify it as a or ẞ. OH HO HO OH HO HO HO OHarrow_forwardWhat are the monomers used to make the following polymers? F. а. b. с. d. Вецер хочому なarrow_forward
- 1. Propose a reasonable mechanism for the following transformation. I'm looking for curved mechanistic arrows and appropriate formal charges on intermediates. OMe MeO OMe Me2N NMe2 OTBS OH xylenes OMe 'OTBSarrow_forwardWhat is the polymer made from the following monomers? What type of polymerization is used for each? а. ОН H2N но b. ن -NH2 d. H₂N NH2 довarrow_forwardCondensation polymers are produced when monomers containing two different functional groups link together with the loss of a small molecule such as H2O. The difunctional monomer H2N(CH2)6COOH forms a condensation polymer. Draw the carbon-skeleton structure of the dimer that forms from this monomer.arrow_forward
- What is the structure of the monomer?arrow_forward→ BINDERIYA GANBO... BINDERIYA GANBO. AP Biology Notes Gamino acid chart - G... 36:22 司 10 ☐ Mark for Review Q 1 Hide 80 8 2 =HA O=A¯ = H₂O Acid HIO HBrO HCIO Question 10 of 35 ^ Σ DELL □ 3 % Λ & 6 7 * ∞ 8 do 5 $ 4 # m 3 ° ( 9 Highlights & Notes AXC Sign out Carrow_forwardWhich representation(s) show polymer structures that are likely to result in rigid, hard materials and those that are likely to result in flexible, stretchable, soft materials?arrow_forward
- 3. Enter the molecular weight of the product obtained from the Williamson Ether Synthesis? OH OH & OH excess CH3l Ag₂Oarrow_forwardPlease answer 1, 2 and 3 on the endarrow_forwardIn the box below, specify which of the given compounds are very soluble in polar aprotic solvents. You may select more than one compound. Choose one or more: NaCl NH4Cl CH3CH2CH2CH2CH2CN CH3CH2OH hexan-2-one NaOH CH3SCH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning