
Concept explainers
(a)
Interpretation: In the titration of glycine hydrochloride (1.0 M and 50 mL) and NaOH, the pH after the addition of 25.0 mL, 50.0 mL and 75.0 mL NaOH needs to be determined.
Concept introduction: For a buffer solution, the pH can be calculated using the Henderson-Hesselbalch equation as follows:
Here, salt is conjugate base of the acid.
(a)
Explanation of Solution
Initially 1.0 M of 50 mL glycine hydrochloride is present. When 25 mL of NaOH is added to it, the following reaction takes place:
The pH of the solution can be calculated using the Henderson-Hesselbalch equation as follows:
Here,
Since, volume of the solution is same, number of moles can be used at the place of concentration.
Now, putting the values,
Thus, the pH when 25 mL of NaOH is added is 2.36.
Now, when 50 mL of NaOH is added the reaction can be represented as follows:
Now, the Zwitter ion can react with water as follows:
Now, total volume is 100 mL thus,
The ICE table can be represented as follows:
The acid dissociation constant can be represented as follows:
Here,
Thus,
Here, acid dissociation constant is very small thus, the value of x can be neglected when compared to 1. Thus,
Now,
Thus, the pH of the solution when 50 mL of NaOH is added is 5.04.
Now, when 75 mL of NaOH is added:
Further reaction takes place:
The pH can be calculated as follows:
Here,
Thus,
or,
Thus, the pH of the solution when 75 mL of NaOH is added is 9.77.
(b)
Interpretation: The titration curve needs to be drawn by indicating the major amino species present after addition of given volume of NaOH.
Concept introduction: For a buffer solution, the pH can be calculated using the Henderson-Hesselbalch equation as follows:
Here, salt is conjugate base of the acid.
(b)
Explanation of Solution
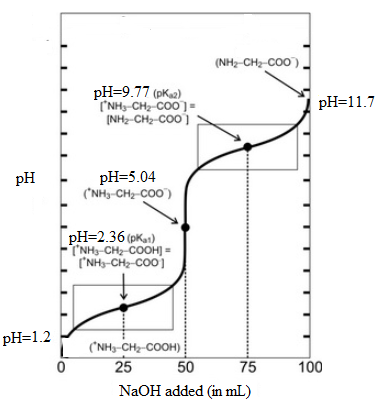
It is assumed that initially the pH is 1.2 and at second equivalent point is 11.7. Here, initial pH means when volume of NaOH is 0.0 mL and second equivalent point means when it is 100 mL. The values can be summarized as follows:
| Volume of NaOH added (mL) | pH |
| 0.0 | 1.2 |
| 25.0 | 2.36 |
| 50.0 | 5.04 |
| 75.0 | 9.77 |
| 100.0 | 11.7 |
Thus, the titration curve indicating the major amino species present at each point will be as follows:
(c)
Interpretation: The pH when the majority of amino acid molecules have net charge equal to zero needs to be determined.
Concept introduction: For a buffer solution, the pH can be calculated using the Henderson-Hesselbalch equation as follows:
Here, salt is conjugate base of the acid.
(c)
Explanation of Solution
The titration curve is as follows:
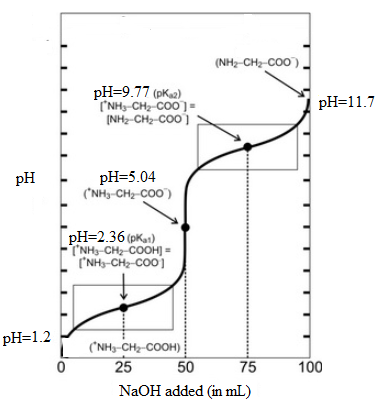
From the above titration curve, it can be seen that when 50 mL of NaOH is added, the majority of amino acid molecules have net charge equal to zero. At this volume corresponds to pH value 5.04. This is known as isoelectric point and denoted as pI and the point on graph corresponds to the equivalent point.
(d)
Interpretation: The pH when the net charge of the major amino acid species is +1/2 and -1/2 needs to be determined.
Concept introduction: For a buffer solution, the pH can be calculated using the Henderson-Hesselbalch equation as follows:
Here, salt is conjugate base of the acid.
(d)
Explanation of Solution
The titration curve is as follows:
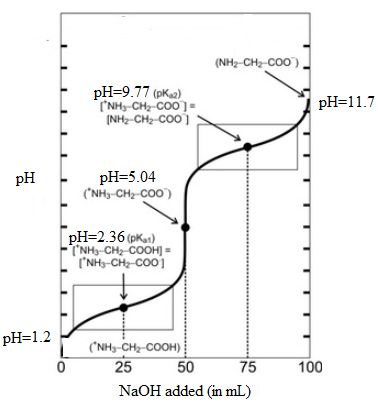
The point when the net charge of the major amino acid species is +1/2 corresponds to first half equivalent point. The volume of NaOH added at this point is 25 mL and corresponding pH is 2.36.
Similarly, the point when the net charge of the major amino acid species is -1/2 corresponds to second half equivalent point. The volume of NaOH added at this point is 75 mL and corresponding pH is 9.77.
Want to see more full solutions like this?
Chapter 21 Solutions
EBK CHEMICAL PRINCIPLES
- 3. Consider the compounds below and determine if they are aromatic, antiaromatic, or non-aromatic. In case of aromatic or anti-aromatic, please indicate number of I electrons in the respective systems. (Hint: 1. Not all lone pair electrons were explicitly drawn and you should be able to tell that the bonding electrons and lone pair electrons should reside in which hybridized atomic orbital 2. You should consider ring strain- flexibility and steric repulsion that facilitates adoption of aromaticity or avoidance of anti- aromaticity) H H N N: NH2 N Aromaticity (Circle) Aromatic Aromatic Aromatic Aromatic Aromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic nonaromatic nonaromatic nonaromatic nonaromatic nonaromatic aromatic TT electrons Me H Me Aromaticity (Circle) Aromatic Aromatic Aromatic Aromatic Aromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic nonaromatic nonaromatic nonaromatic nonaromatic nonaromatic aromatic πT electrons H HH…arrow_forwardA chemistry graduate student is studying the rate of this reaction: 2 HI (g) →H2(g) +12(g) She fills a reaction vessel with HI and measures its concentration as the reaction proceeds: time (minutes) [IH] 0 0.800M 1.0 0.301 M 2.0 0.185 M 3.0 0.134M 4.0 0.105 M Use this data to answer the following questions. Write the rate law for this reaction. rate = 0 Calculate the value of the rate constant k. k = Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol.arrow_forwardNonearrow_forward
- in which spectral range of EMR, atomic and ionic lines of metal liesarrow_forwardQ2: Label the following molecules as chiral or achiral, and label each stereocenter as R or S. CI CH3 CH3 NH2 C CH3 CH3 Br CH3 X &p Bra 'CH 3 "CH3 X Br CH3 Me - N OMe O DuckDuckarrow_forward1. For the four structures provided, Please answer the following questions in the table below. a. Please draw π molecular orbital diagram (use the polygon-and-circle method if appropriate) and fill electrons in each molecular orbital b. Please indicate the number of π electrons c. Please indicate if each molecule provided is anti-aromatic, aromatic, or non- aromatic TT MO diagram Number of π e- Aromaticity Evaluation (X choose one) Non-aromatic Aromatic Anti-aromatic || ||| + IVarrow_forward
- 1.3 grams of pottasium iodide is placed in 100 mL of o.11 mol/L lead nitrate solution. At room temperature, lead iodide has a Ksp of 4.4x10^-9. How many moles of precipitate will form?arrow_forwardQ3: Circle the molecules that are optically active: ДДДДarrow_forward6. How many peaks would be observed for each of the circled protons in the compounds below? 8 pts CH3 CH3 ΤΙ A. H3C-C-C-CH3 I (₁₁ +1)= 7 H CI B. H3C-C-CI H (3+1)=4 H LIH)=2 C. (CH3CH2-C-OH H D. CH3arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





