
EBK CHEMICAL PRINCIPLES
8th Edition
ISBN: 9781305856745
Author: DECOSTE
Publisher: CENGAGE LEARNING - CONSIGNMENT
expand_more
expand_more
format_list_bulleted
Question
Chapter 21, Problem 35E
Interpretation Introduction
Interpretation: The groups −COOH and −CHO and R in both carboxylic acids and
Concept introduction: Organic molecules have some structural features in addition to
Table given below gives information about the carboxylic and aldehyde functional group:
| Type of Compound | General Structure |
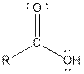 | |
| Aldehyde | 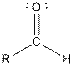 |
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What does the phrase 'fit for purpose' mean in relation to analytical chemistry? Please provide examples too.
For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the
benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene.
Molecule
Inductive Effects
Resonance Effects
Overall Electron-Density
×
NO2
○ donating
O donating
O withdrawing
O withdrawing
O electron-rich
electron-deficient
no inductive effects
O no resonance effects
O similar to benzene
E
[
CI
O donating
withdrawing
O no inductive effects
Explanation
Check
○ donating
withdrawing
no resonance effects
electron-rich
electron-deficient
O similar to benzene
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Acces
Understanding how substituents activate
Rank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic
aromatic substitution.
Explanation
HN
NH2
Check
X
(Choose one)
(Choose one)
(Choose one)
(Choose one)
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center A
Chapter 21 Solutions
EBK CHEMICAL PRINCIPLES
Ch. 21 - Prob. 1ECh. 21 - Prob. 2ECh. 21 - Why are cyclopropane and cyclobutane so reactive?Ch. 21 - Prob. 4ECh. 21 - Prob. 5ECh. 21 - Prob. 6ECh. 21 - Prob. 7ECh. 21 - Name the five structural isomers of C6H14 .Ch. 21 - Draw the structural formula for each of the...Ch. 21 - Prob. 10E
Ch. 21 - Prob. 11ECh. 21 - Name each of the following cyclic alkanes, and...Ch. 21 - Prob. 13ECh. 21 - Prob. 14ECh. 21 - Prob. 15ECh. 21 - Prob. 16ECh. 21 - Prob. 17ECh. 21 - Prob. 18ECh. 21 - Prob. 19ECh. 21 - Prob. 20ECh. 21 - Prob. 21ECh. 21 - Prob. 22ECh. 21 - Prob. 23ECh. 21 - Prob. 24ECh. 21 - Prob. 25ECh. 21 - Prob. 26ECh. 21 - Prob. 27ECh. 21 - Prob. 28ECh. 21 - Prob. 29ECh. 21 - Prob. 30ECh. 21 - Name the following compounds.Ch. 21 - Prob. 32ECh. 21 - Prob. 33ECh. 21 - Prob. 34ECh. 21 - Prob. 35ECh. 21 - Prob. 36ECh. 21 - Prob. 37ECh. 21 - Prob. 38ECh. 21 - Prob. 39ECh. 21 - Prob. 40ECh. 21 - Prob. 41ECh. 21 - Draw structural formulas for each of the following...Ch. 21 - Prob. 43ECh. 21 - Prob. 44ECh. 21 - Prob. 45ECh. 21 - Prob. 46ECh. 21 - Prob. 47ECh. 21 - Prob. 48ECh. 21 - Prob. 49ECh. 21 - Prob. 50ECh. 21 - Prob. 51ECh. 21 - Prob. 52ECh. 21 - Prob. 53ECh. 21 - Prob. 54ECh. 21 - Prob. 55ECh. 21 - Prob. 56ECh. 21 - Prob. 57ECh. 21 - Prob. 58ECh. 21 - Prob. 59ECh. 21 - Give an example reaction that would yield the...Ch. 21 - Prob. 61ECh. 21 - Prob. 62ECh. 21 - Prob. 63ECh. 21 - Prob. 64ECh. 21 - Prob. 65ECh. 21 - Prob. 66ECh. 21 - Prob. 67ECh. 21 - Prob. 68ECh. 21 - Prob. 69ECh. 21 - Prob. 70ECh. 21 - Prob. 71ECh. 21 - Prob. 72ECh. 21 - Prob. 73ECh. 21 - Prob. 74ECh. 21 - Prob. 75ECh. 21 - Prob. 76ECh. 21 - Prob. 77ECh. 21 - Prob. 78ECh. 21 - Prob. 79ECh. 21 - Prob. 80ECh. 21 - Prob. 81ECh. 21 - Prob. 82ECh. 21 - Prob. 83ECh. 21 - Prob. 84ECh. 21 - Prob. 85ECh. 21 - Prob. 86ECh. 21 - Prob. 87ECh. 21 - Prob. 88ECh. 21 - Prob. 89ECh. 21 - Prob. 90ECh. 21 - Prob. 91ECh. 21 - Prob. 92ECh. 21 - Prob. 93ECh. 21 - Prob. 94ECh. 21 - Prob. 95ECh. 21 - Draw the structures of the tripeptides gly-ala-ser...Ch. 21 - Prob. 97ECh. 21 - Prob. 98ECh. 21 - What types of interactions can occur between the...Ch. 21 - Prob. 100ECh. 21 - Prob. 101ECh. 21 - Prob. 102ECh. 21 - Prob. 103ECh. 21 - Prob. 104ECh. 21 - Prob. 105ECh. 21 - Prob. 106ECh. 21 - Prob. 107ECh. 21 - Prob. 108ECh. 21 - Prob. 109ECh. 21 - Prob. 110ECh. 21 - Prob. 111ECh. 21 - Prob. 112ECh. 21 - Prob. 113ECh. 21 - Prob. 114ECh. 21 - Prob. 115ECh. 21 - Prob. 116ECh. 21 - Prob. 117ECh. 21 - Prob. 118ECh. 21 - Prob. 119ECh. 21 - Prob. 120ECh. 21 - Prob. 121ECh. 21 - Prob. 122ECh. 21 - Prob. 123ECh. 21 - Prob. 124ECh. 21 - Prob. 125ECh. 21 - Prob. 126ECh. 21 - Prob. 127AECh. 21 - Prob. 128AECh. 21 - Prob. 129AECh. 21 - Prob. 130AECh. 21 - Prob. 131AECh. 21 - Prob. 132AECh. 21 - Prob. 133AECh. 21 - Prob. 134AECh. 21 - Prob. 135AECh. 21 - Prob. 136AECh. 21 - Prob. 137AECh. 21 - Prob. 138AECh. 21 - Prob. 139AECh. 21 - Prob. 140AECh. 21 - Prob. 141AECh. 21 - Prob. 142AECh. 21 - Prob. 143AECh. 21 - Prob. 144AECh. 21 - Prob. 145AECh. 21 - Prob. 146AECh. 21 - Prob. 147AECh. 21 - Prob. 148AECh. 21 - Prob. 149AECh. 21 - Prob. 150AECh. 21 - Prob. 151AECh. 21 - Prob. 152AECh. 21 - Prob. 153AECh. 21 - Prob. 154AECh. 21 - Prob. 155AECh. 21 - Prob. 156AECh. 21 - Prob. 157AECh. 21 - Prob. 158AECh. 21 - Prob. 159AECh. 21 - Prob. 160AECh. 21 - Prob. 161AECh. 21 - Name each of the following cyclic alkanes.Ch. 21 - Prob. 163AECh. 21 - Prob. 164AECh. 21 - Prob. 165AECh. 21 - Prob. 166AECh. 21 - Prob. 167AECh. 21 - Prob. 168AECh. 21 - Prob. 169CPCh. 21 - Prob. 170CPCh. 21 - Prob. 171CPCh. 21 - Prob. 172CPCh. 21 - Prob. 173CPCh. 21 - Prob. 174CP
Knowledge Booster
Similar questions
- Identifying electron-donating and electron-withdrawing effects on benzene For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Inductive Effects Resonance Effects Overall Electron-Density Molecule CF3 O donating O donating O withdrawing O withdrawing O no inductive effects O no resonance effects electron-rich electron-deficient O similar to benzene CH3 O donating O withdrawing O no inductive effects O donating O withdrawing Ono resonance effects O electron-rich O electron-deficient O similar to benzene Explanation Check Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward* Hint: Think back to Chem 1 solubility rules. Follow Up Questions for Part B 12. What impact do the following disturbances to a system at equilibrium have on k, the rate constant for the forward reaction? Explain. (4 pts) a) Changing the concentration of a reactant or product. (2 pts) b) Changing the temperature of an exothermic reaction. (2 pts) ofarrow_forwardDraw TWO general chemical equation to prepare Symmetrical and non-Symmetrical ethers Draw 1 chemical reaction of an etherarrow_forward
- Give the chemical equation for the preparation of: -Any aldehyde -Any keytonearrow_forward+ C8H16O2 (Fatty acid) + 11 02 → 8 CO2 a. Which of the above are the reactants? b. Which of the above are the products? H2o CO₂ c. Which reactant is the electron donor? Futty acid d. Which reactant is the electron acceptor? e. Which of the product is now reduced? f. Which of the products is now oxidized? 02 #20 102 8 H₂O g. Where was the carbon initially in this chemical reaction and where is it now that it is finished? 2 h. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forward→ Acetyl-CoA + 3NAD+ + 1FAD + 1ADP 2CO2 + CoA + 3NADH + 1FADH2 + 1ATP a. Which of the above are the reactants? b. Which of the above are the products? c. Which reactant is the electron donor? d. Which reactants are the electron acceptors? e. Which of the products are now reduced? f. Which product is now oxidized? g. Which process was used to produce the ATP? h. Where was the energy initially in this chemical reaction and where is it now that it is finished? i. Where was the carbon initially in this chemical reaction and where is it now that it is finished? j. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forward
- Rank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. OCH 3 (Choose one) OH (Choose one) Br (Choose one) Explanation Check NO2 (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Aarrow_forwardFor each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects O donating O withdrawing O no inductive effects Resonance Effects Overall Electron-Density ○ donating ○ withdrawing O no resonance effects O electron-rich O electron-deficient O similar to benzene Cl O donating O withdrawing ○ donating ○ withdrawing O no inductive effects O no resonance effects O Explanation Check O electron-rich O electron-deficient similar to benzene X © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forwardIdentifying electron-donating and For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects NH2 ○ donating NO2 Explanation Check withdrawing no inductive effects Resonance Effects Overall Electron-Density ○ donating O withdrawing O no resonance effects O donating O withdrawing O donating withdrawing O no inductive effects Ono resonance effects O electron-rich electron-deficient O similar to benzene O electron-rich O electron-deficient O similar to benzene olo 18 Ar 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning