
a)
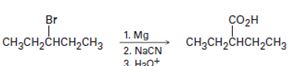
Interpretation:
The flaw in the synthetic scheme given is to be identified.
Concept introduction:
To identify:
The flaw in the synthetic scheme given.
b)
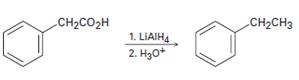
Interpretation:
The flaw in the synthetic scheme given is to be identified.
Concept introduction:
Carboxylic acids are reduced to alcohols and not to
To identify:
The flaw in the synthetic scheme given.
c)
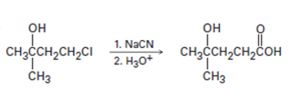
Interpretation:
The flaw in the synthetic scheme given is to be identified.
Concept introduction:
Alkyl halides when treated with cyanides yield nitriles which upon hydrolysis give carboxylic acids. Tertiary alcohols get dehydrate to yield
To identify:
The flaw in the synthetic scheme given.
Trending nowThis is a popular solution!

Chapter 20 Solutions
Organic Chemistry
- 3. SYNTHESIS. Propose a sequence of synthetic steps (FGI) that convert the starting material (SM) into the Target molecule. For each FGI in your proposed synthesis, specify the reagents / conditions, and draw the product(s) of that FGI. DO NOT INCLUDE the FGI mxn in the answer you submit. If an FGI requires two reagent sets, specify the order in which the reagent sets are added, e.g., i) Hg(OAc)2 / H₂O; ii) NaBH4/MeOH. Indicate the stereochemistry (if any) of the products of each FGI. FGI 1. Me Starting Material Source of all carbons in the Target molecule (can use multiple copies) Me Me Target molecule + enantiomerarrow_forwardcurved arrows are used to illustate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction mechanism stepsarrow_forwardIf is was a very hot day, what would the aldol condensation product be? *see imagearrow_forward
- Please help me with number 1-3. Thank you so much.arrow_forwardDraw the major product of this reaction ingnore the inorganic byproducts. 1. NaOCH2CH3 at 25 C 2. PhCH2Br (1 eq)arrow_forwardAt 90ºC the vapor pressure of ortho-xylene is 20 kPa and that of meta-xylene is 18 kPa. What is the composition of the vapor in equilibrium with a mixture in which the mole fraction of o-xylene is 0.60?arrow_forward
- Draw the products of this reduction of a ketone with sodium borohydride. Use a dash or wedge bond to indicate the stereochemistry of substituents on asymmetric centers, where applicableIgnore any inorganic byproducts. 1) NaBH4 2) HCI/H2O Select to Drawarrow_forwardWhy do you think people who live at high altitudes are advised to add salt to water when boiling food like pasta? What mole fraction of NaCl is needed to raise the boiling point of H2O by 3˚C? Does the amount of salt added to water (typically about one teaspoon to four quarts of water) substantially change the boiling point? (Kb (H2O) = 0.51˚C/molal.)arrow_forwardpls help asaparrow_forward
- pls help asaparrow_forward9. Consider the following galvanic cell: Fe (s) | Fe(NO3)2 (aq) || Sn(NO3)2 (aq) | Sn (s) a. Write an equation for the half reactions occurring at the anode and cathode. b. Calculate the standard cell potential Show all of your work. c. Draw and label the galvanic cell, including the anode and cathode, direction of electron flow, and direction of ion migration.arrow_forwardpls help asaparrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


