
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20.SE, Problem 64AP
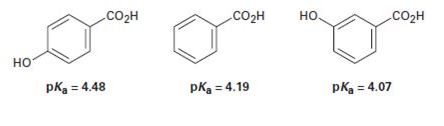
The following pKa values have been measured. Explain why a hydroxyl group in the para position decreases the acidity while a hydroxyl group in the meta position increases the acidity.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
13
How many signals would you expect to see in the
Check
O
signal(s)
X
§
'C NMR spectrum for the following compound?
© 2025 McGraw Hill
13
Consider the "C NMR spectrum below.
140
120
100
80
60
40
20
20
PPM
0
The spectrum belongs to which one of the following constitutional isomers of the compound C,H12? Select the single best answer.
Check
✓
G
Save For Later
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use
The structure of compound 1,3,5-trimethylbenzene (mesitylene) is given below.
How many signals would you expect to find in the 'H NMR spectrum of 1,3,5-trimethylbenzene (mesitylene)?
Check
×
Chapter 20 Solutions
Organic Chemistry
Ch. 20.1 - Give IUPAC names for the following compounds:Ch. 20.1 - Draw structures corresponding to the following...Ch. 20.2 - Prob. 3PCh. 20.2 - The Ka for dichloroacetic acid is 3.32 Ă— 10-2....Ch. 20.3 - Calculate the percentages of dissociated and...Ch. 20.4 - Which would you expect to be a stronger acid, the...Ch. 20.4 - Dicarboxylic acids have two dissociation...Ch. 20.4 - The pKa of p-cyclopropylbenzoic acid is 4.45. Is...Ch. 20.4 - Prob. 9PCh. 20.5 - Prob. 10P
Ch. 20.6 - Prob. 11PCh. 20.6 - How might you carry out the following...Ch. 20.7 - Prob. 13PCh. 20.7 - Prob. 14PCh. 20.8 - Cyclopentanecarboxylic acid and...Ch. 20.8 - Prob. 16PCh. 20.SE - Prob. 17VCCh. 20.SE - Prob. 18VCCh. 20.SE - The following carboxylic acid can’t be prepared...Ch. 20.SE - Electrostatic potential maps of anisole and...Ch. 20.SE - Predict the product(s) and provide the mechanism...Ch. 20.SE - Predict the product(s) and provide the mechanism...Ch. 20.SE - Prob. 23MPCh. 20.SE - Predict the product(s) and provide the complete...Ch. 20.SE - Acid-catalyzed hydrolysis of a nitrile to give a...Ch. 20.SE - Prob. 26MPCh. 20.SE - Naturally occurring compounds called cyanogenic...Ch. 20.SE - 2-Bromo-6, 6-dimethylcyclohexanone gives 2,...Ch. 20.SE - Naturally occurring compounds called terpenoids,...Ch. 20.SE - In the Ritter reaction, an alkene reacts with a...Ch. 20.SE - Give IUPAC names for the following compounds:Ch. 20.SE - Prob. 32APCh. 20.SE - Prob. 33APCh. 20.SE - Prob. 34APCh. 20.SE - Prob. 35APCh. 20.SE - Prob. 36APCh. 20.SE - Prob. 37APCh. 20.SE - Prob. 38APCh. 20.SE - Calculate the Ka's for the following acids: (a)...Ch. 20.SE - Thioglycolic acid, HSCH2CO2H, a substance used in...Ch. 20.SE - Prob. 41APCh. 20.SE - Prob. 42APCh. 20.SE - How could you convert butanoic acid into the...Ch. 20.SE - How could you convert each of the following...Ch. 20.SE - How could you convert butanenitrile into the...Ch. 20.SE - How would you prepare the following compounds from...Ch. 20.SE - Prob. 47APCh. 20.SE - Using 13CO2 as your only source of labeled carbon,...Ch. 20.SE - Prob. 49APCh. 20.SE - Which method-Grignard carboxylation or nitrile...Ch. 20.SE - Prob. 51APCh. 20.SE - Prob. 52APCh. 20.SE - Propose a structure for a compound C6H12O2 that...Ch. 20.SE - Prob. 54APCh. 20.SE - How would you use NMR (either 13C or 1H) to...Ch. 20.SE - Prob. 56APCh. 20.SE - A chemist in need of 2,2-dimethylpentanoic acid...Ch. 20.SE - Prob. 58APCh. 20.SE - Prob. 59APCh. 20.SE - Prob. 60APCh. 20.SE - Prob. 61APCh. 20.SE - Prob. 62APCh. 20.SE - Prob. 63APCh. 20.SE - The following pKa values have been measured....Ch. 20.SE - Identify the missing reagents a-f in the following...Ch. 20.SE - Propose a structure for a compound, C4H7N, that...Ch. 20.SE - Prob. 67APCh. 20.SE - The 1H and 13C NMR spectra below belong to a...Ch. 20.SE - Propose structures for carboxylic acids that show...Ch. 20.SE - Carboxylic acids having a second carbonyl group...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1 How many signals do you expect in the 'H NMR spectrum for this molecule? CI CI Cl Write the answer in the table below. Also, in each of the drawing areas below is a copy of the molecule, with H atoms shown. In each copy, one of the H atoms is highlighted red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red. Note for advanced students: Remember, a multiplet is considered one signal in the 'H NMR spectrum. 1 Number of signals in the 'H NMR spectrum. ☐ For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional H atoms to highlight in top molecule For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at…arrow_forwardwrtie the balanced equation and find the E° when the following half- reactions are combined Zn2+(aq) + 2e---> Zn(s) E°= -0.763V Ag+(aq) + e---> Ag (s) E°=+0.799Varrow_forwardConsider this molecule: How many H atoms are in this molecule? How many different signals could be found in its 'H NMR spectrum? Note: A multiplet is considered one signal. ☐arrow_forward
- Study this 'H NMR spectrum, and then answer the questions about it in the table below. Check 1.0- 0.5- 0.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 What unit symbol should be written on the horizontal axis? What is the chemical shift & of the doublet? If there is no doublet, just check the box instead. Give your answer to 2 significant digits. What is the chemical shift of the signal immediately upfield of the doublet? If there is no doublet, or no signal upfield of it, check the box instead. What is the chemical shift & of the least deshielded proton? If you can't tell without more information, check the box instead. 血 8 = ☐ There is no doublet. 8 = ☐ No such signal. 8 = 0 Need more information.arrow_forwardhow many moles of H2O2 are required to react with 11g of N2H4 according to the following reaction? (atomic weights: N=14.01, H=1.008, O= 16.00) 7H2O2 + N2H4 -> 2HNO3 + 8H20arrow_forwardcalculate the number of moles of H2 produced from 0.78 moles of Ga and 1.92 moles HCL? 2Ga+6HCL->2GaCl3+3H2arrow_forward
- an adult human breathes 0.50L of air at 1 atm with each breath. If a 50L air tank at 200 atm is available, how man y breaths will the tank providearrow_forwardWhat are the advantages and/or disadvantages of using the MOHR titration method & AOEC method?arrow_forwardAre there any alternative methods better than the MOHR titration to quantitatively determine salt in a sample?arrow_forward
- hybridization of nitrogen of complex moleculesarrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NO2 (g) = N2O4(g) AGº = -5.4 kJ Now suppose a reaction vessel is filled with 4.53 atm of dinitrogen tetroxide (N2O4) at 279. °C. Answer the following questions about this system: Under these conditions, will the pressure of N2O4 tend to rise or fall? Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to '2' rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO 2 needed to reverse it. Round your answer to 2 significant digits. 00 rise ☐ x10 fall yes no ☐ atm G Ar 1arrow_forwardWhy do we analyse salt?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License