
Concept explainers
a) cis-1,2-Cyclohexanedicarboxylic acid
Interpretation:
The structure of cis-1,2-cyclohexanedicarboxylic acid is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain
To give:
The structure of cis-1,2-cyclohexanedicarboxylic acid.
Answer to Problem 32AP
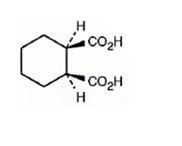
The structure of cis-1,2-cyclohexanedicarboxylic acid is
Explanation of Solution
The name shows that the compound has a cyclohexane ring with two carboxylic acid groups in 1,2 positions arranged in the same side of the ring.
The structure of cis-1,2-cyclohexanedicarboxylic acid is

b) Heptanedioic acid
Interpretation:
The structure of heptanedioic acid is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain alkanes are arrived by replacing the terminal –e of the corresponding alkane name by –oic acid. The numbering starts from carboxyl carbon. Compounds with –COOH bonded to a ring are named using the suffix-carboxylic acid. The –COOH carbon in this case is not numbered as C1, instead the carbon to which it is attached is numbered as C1. As a substituent, the –COOH group is called as carboxyl group.
To give:
The structure of heptanedioic acid.
Answer to Problem 32AP
The structure of heptanedioic acid is
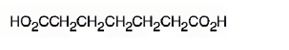
Explanation of Solution
The name shows that the compound has a seven carbon straight chain with two carboxyl groups at theb ends.
The structure of heptanedioic acid is
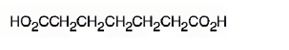
c) 2-Hexen-4-ynoic acid
Interpretation:
The structure of 2-hexen-4-ynoic acid is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain alkanes are arrived by replacing the terminal –e of the corresponding alkane name by –oic acid. The numbering starts from carboxyl carbon. Compounds with –COOH bonded to a ring are named using the suffix-carboxylic acid. The –COOH carbon in this case is not numbered as C1, instead the carbon to which it is attached is numbered as C1. As a substituent, the –COOH group is called as carboxyl group.
To give:
The structure of 2-hexen-4-ynoic acid.
Answer to Problem 32AP
The structure of 2-hexen-4-ynoic acid is
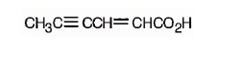
Explanation of Solution
The name shows that the compound has a six carbon straight chain with a carboxylic group, a double bond between C2 & C3 and a triple bond between C4 & C5.
The structure of 2-hexen-4-ynoic acid is
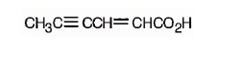
d) 4-Ethyl-2-propyloctanoic acid
Interpretation:
The structure of 4-ethyl-2-propyloctanoic acid is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain alkanes are arrived by replacing the terminal –e of the corresponding alkane name by –oic acid. The numbering starts from carboxyl carbon. Compounds with –COOH bonded to a ring are named using the suffix-carboxylic acid. The –COOH carbon in this case is not numbered as C1, instead the carbon to which it is attached is numbered as C1. As a substituent, the –COOH group is called as carboxyl group.
To give:
The structure of 4-ethyl-2-propyloctanoic acid.
Answer to Problem 32AP
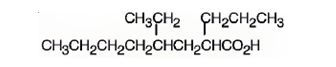
The structure of 4-ethyl-2-propyloctanoic acid is
Explanation of Solution
The name shows that the compound is an octane derivative and has a carboxyl group, a propyl group on C2 and an ethyl group on C4.
The structure of 4-ethyl-2-propyloctanoic acid is

e) 3-Chlorophthalic acid
Interpretation:
The structure of 3-chlorophthalic acid is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain alkanes are arrived by replacing the terminal –e of the corresponding alkane name by –oic acid. The numbering starts from carboxyl carbon. Compounds with –COOH bonded to a ring are named using the suffix-carboxylic acid. The –COOH carbon in this case is not numbered as C1, instead the carbon to which it is attached is numbered as C1. As a substituent, the –COOH group is called as carboxyl group.
To give:
The structure of 3-chlorophthalic acid.
Answer to Problem 32AP
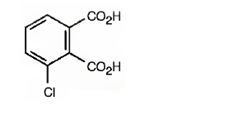
The structure of 3-chlorophthalic acid is
Explanation of Solution
The name indicates that the compound is a benzene derivative with two carboxyl groups on C1 & C2 and a chlorine atom on C3.
The structure of 3-chlorophthalic acid is

f) Triphenylacetic acid
Interpretation:
The structure of triphenylacetic acid is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain alkanes are arrived by replacing the terminal –e of the corresponding alkane name by –oic acid. The numbering starts from carboxyl carbon. Compounds with –COOH bonded to a ring are named using the suffix-carboxylic acid. The –COOH carbon in this case is not numbered as C1, instead the carbon to which it is attached is numbered as C1. As a substituent, the –COOH group is called as carboxyl group.
To give:
The structure of triphenylacetic acid.
Answer to Problem 32AP
The structure of triphenylacetic acid is
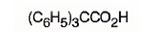
Explanation of Solution
The structure of acetic acid is CH3COOH. The name of the compound given indicates that it has three phenyl groups instead of the three hydrogen atoms present in methyl group in acetic acid.
The structure of triphenylacetic acid is
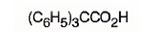
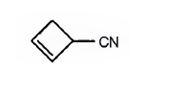
g) 2-Cyclobutenecarbonitrile
Interpretation:
The structure of 2-cyclobutenecarbonitrile is to be given.
Concept introduction:
Simple open chain nitriles are named by adding –nitrile as suffix to the alkane name, with the nitrile carbon numbered as C1. Nitriles can also be names as derivatives of carboxylic acids by replacing the –ic acid or –oic acid ending with –onitrile. The nitrile carbon is not numbered but the carbon to which it is attached is numbered ac C1. If another carboxylic acid derivative is present in the same molecule, the prefix –cyano is used for the –CN group.
To give:
The structure of 2-cyclobutenecarbonitrile.
Answer to Problem 32AP
The structure of 2-cyclobutenecarbonitrile is
Explanation of Solution
The name shows that the compound has a nitrile group attached to a cyclobutene ring with a double bond between C2 & C3.
The structure of 2-cyclobutenecarbonitrile is

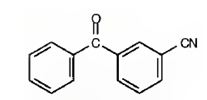
h) m-Benzoylbenzonitrile
Interpretation:
The structure of m-benzoylbenzonitrile is to be given.
Concept introduction:
Simple open chain nitriles are named by adding –nitrile as suffix to the alkane name, with the nitrile carbon numbered as C1. Nitriles can also be names as derivatives of carboxylic acids by replacing the –ic acid or –oic acid ending with –onitrile. The nitrile carbon is not numbered but the carbon to which it is attached is numbered ac C1. If another carboxylic acid derivative is present in the same molecule, the prefix –cyano is used for the –CN group.
To give:
The structure of m-benzoylbenzonitrile.
Answer to Problem 32AP
The structure of m-benzoylbenzonitrile is
Explanation of Solution
The name shows that the compound has a benzene ring attached to a nitrile group and a benzoyl group with meta relationship.
The structure of m-benzoylbenzonitrile is

Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry
- Predict the intermediate 1 and final product 2 of this organic reaction: NaOMe H+ + 1 2 H H work up You can draw 1 and 2 in any arrangement you like. Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center. Click and drag to start drawing a structure. X $ dmarrow_forwardPredict the major products of this organic reaction: 1. NaH (20°C) 2. CH3Br ? Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. G Crarrow_forwardPredict the major products of this organic reaction: 1. LDA (-78°C) ? 2. Br Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. . • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. Xarrow_forward
- Please draw the structuresarrow_forwardDraw the missing intermediates 1 and 2, plus the final product 3, of this synthesis: 0 1. Eto 1. Eto- 1 2 2. MeBr 2. EtBr H3O+ A 3 You can draw the three structures in any arrangement you like. Explanation Check Click and drag to start drawing a structure.arrow_forwardDraw the missing intermediate 1 and final product 2 of this synthesis: 1. MeO- H3O+ 1 2 2. PrBr Δ You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forward
- What is the differences between: Glyceride and phosphoglyceride Wax and Fat Soap and Fatty acid HDL and LDL cholesterol Phospho lipids and sphingosine What are the types of lipids? What are the main lipid components of membrane structures? How could lipids play important rules as signaling molecules and building units? The structure variety of lipids makes them to play significant rules in our body, conclude breifly on this statement.arrow_forwardWhat is the differences between DNA and RNA for the following: - structure - function - type What is the meaning of: - replication - transcription - translation show the base pair connection(hydrogen bond) in DNA and RNAarrow_forwardWhat is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward
- What is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward> aw the missing intermediates 1 and 2, plus the final product 3, of this synthesis: 1. Eto 1. EtO¯ H3O+ 1 2 2. PrBr 2. PrBr Δ You can draw the three structures in any arrangement you like. 3 Click and drag to start drawing a structure. Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacarrow_forwardThere are various factors that affect an equilibrium. Give 3 of these factors and explain using examples andequations how an equilibrium is affected by these factors. Please remember that this is a communication question so that you are communicating your understanding of the factors that affect and equilibrium.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

