
Concept explainers
(a)
Interpretation:
The spatial arrangement for the
Concept Introduction:
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and
Saturated hydrocarbons are
Alkane has general molecular formula as
Considering the geometry of carbon atoms, the carbon atoms that have double bonds will have trigonal planar geometry. The carbon atoms that have only single bonds attached to it will have tetrahedral geometry. The carbon atoms that have a triple bond attached to it will have a linear geometry.
(a)
Answer to Problem 2.37EP
The spatial arrangement is identified as tetrahedral.
Explanation of Solution
Given structure is,

Looking into the left most carbon atom present in the given structure, it is not bonded to any double bonds or triple bond. This carbon atom has only four single bonds (three with hydrogen and one with carbon atom). Therefore, the spatial arrangement of the left-most carbon atom is tetrahedral.
The spatial arrangement of the left-most carbon atom is identified.
(b)
Interpretation:
The spatial arrangement for the chemical bonds in the left‑most carbon atom in the given structure has to be identified.
Concept Introduction:
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Saturated hydrocarbons are alkanes. Unsaturated hydrocarbons are alkene, alkyne and aromatic hydrocarbons.
Alkane has general molecular formula as
Considering the geometry of carbon atoms, the carbon atoms that have double bonds will have trigonal planar geometry. The carbon atoms that have only single bonds attached to it will have tetrahedral geometry. The carbon atoms that have a triple bond attached to it will have a linear geometry.
(b)
Answer to Problem 2.37EP
The spatial arrangement is identified as tetrahedral.
Explanation of Solution
Given structure is,

Looking into the left most carbon atom present in the given structure, it is not bonded to any double bonds or triple bond. This carbon atom has only four single bonds (three with hydrogen and one with carbon atom). Therefore, the spatial arrangement of the left-most carbon atom is tetrahedral.
The spatial arrangement of the left-most carbon atom is identified.
(c)
Interpretation:
The spatial arrangement for the chemical bonds in the left‑most carbon atom in the given structure has to be identified.
Concept Introduction:
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Saturated hydrocarbons are alkanes. Unsaturated hydrocarbons are alkene, alkyne and aromatic hydrocarbons.
Alkane has general molecular formula as
Considering the geometry of carbon atoms, the carbon atoms that have double bonds will have trigonal planar geometry. The carbon atoms that have only single bonds attached to it will have tetrahedral geometry. The carbon atoms that have a triple bond attached to it will have a linear geometry.
(c)
Answer to Problem 2.37EP
The spatial arrangement is identified as trigonal planar.
Explanation of Solution
Given structure is,

Looking into the left most carbon atom present in the given structure, it is bonded to one double bond. This carbon atom has only two single bonds with hydrogen and a double bond with carbon atom. Therefore, the spatial arrangement of the left-most carbon atom is trigonal planar.
The spatial arrangement of the left-most carbon atom is identified.
(d)
Interpretation:
The spatial arrangement for the chemical bonds in the left‑most carbon atom in the given structure has to be identified.
Concept Introduction:
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Saturated hydrocarbons are alkanes. Unsaturated hydrocarbons are alkene, alkyne and aromatic hydrocarbons.
Alkane has general molecular formula as
Considering the geometry of carbon atoms, the carbon atoms that have double bonds will have trigonal planar geometry. The carbon atoms that have only single bonds attached to it will have tetrahedral geometry. The carbon atoms that have a triple bond attached to it will have a linear geometry.
(d)
Answer to Problem 2.37EP
The spatial arrangement is identified as tetrahedral.
Explanation of Solution
Given structure is,
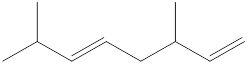
Looking into the left most carbon atom present in the given structure, it is not bonded to any double bonds or triple bond. This carbon atom has only four single bonds (three with hydrogen and one with carbon atom). Therefore, the spatial arrangement of the left-most carbon atom is tetrahedral.
The spatial arrangement of the left-most carbon atom is identified.
Want to see more full solutions like this?
Chapter 2 Solutions
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
- Make meta-dibromobenze from nitrobenzene using amine reactions. *see imagearrow_forwardProvide the structure of the expected major and minor (if any) products for each reaction. Clearly indicate stereochemistry where warranted. + + heat heat 이요 HNO3 1. AlCl3 2. H₂O H2SO4 1. AlCl3arrow_forward) Give the mechanism for the acid catalyzed hydrolysis of the following to the corresponding carboxylic acid. Show all intermediates and resonance structures N H+, H2O (excess)arrow_forward
- # 2. Drow full structures of the organic product expected in each of the following reactions. Draw the appropriate stereoisomer where warranted! Tos Cl O C NaCN PCC శ్రీ CI TSCI Pyridine H₂CrO4 PBrj Pyridine NaCNarrow_forwardPLEASE help. Locate a literature IR spectrum of eugenol. Insert the literature spectrum here: What conclusions can you draw about your clove oil from these IR spectra? I attached my data belowarrow_forwardplease help and the percent recovery of clove oil from cloves is 4.61% and i have attached my ir spectrum as well. Based on your GC data, how many components are in the clove oil? Calculate the percentage of each component. Clearly show your work. Which of the components corresponds to eugenol? How do you know? Is eugenol the major component?arrow_forward
- please help and i am so confused if the picture is the gc data or ir spectrum. you dont have to do everything just what you can please because i am lost and the mass of the cloves was Mass of cloves 62.299g. Mass of recovered clove oil 62.761g.arrow_forwardWhich compound would you expect to have a higher decomposition temperature,Na2CO3 or Cs2CO3? Justify your answer, but you do not need to do any calculations.arrow_forwardCan I get some help drawing my arrows. I included what the final needs to look likearrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





