
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 1.SE, Problem 45AP
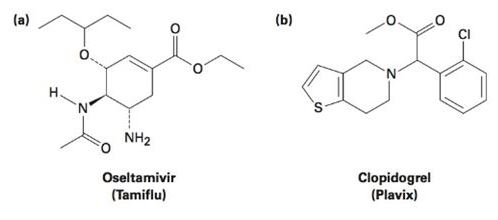
Tell the number of hydrogens bonded to each carbon atom in (a) the antiinfluenza agent oseltamivir, marketed as Tamiflu, and (b) the platelet aggregation inhibitor clopidogrel, marketed as Plavix. Give the molecular formula of each.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
5. Compressibility (6 points total). The isothermal compressibility is a measure of how
hard/easy it is to compress an object (how squishy is it?) at constant temperature. It is
др
defined as Br=-()=-(200²)T'
(a) You might wonder why there is a negative sign in this formula. What does it mean when
this quantity is positive and what does it mean when this quantity is negative?
(b) Derive the formula for the isothermal compressibility of an ideal gas (it is very simple!)
(c) Explain under what conditions for the ideal gas the compressibility is higher or lower,
and why that makes sense.
19. (3 pts) in Chapter 7 we will see a reaction of halocyclohexanes that requires that the halogen occupy an axial position with
this in mind, would you expect cis-1-bromo-3-methylcyclohexane or trans-1-bromo-3-methylcyclohexane to be more
reactive in this reaction? Briefly explain your choice using structures to support your answer.
Mere-eries-cecleone)
The tran-i-browse-3-methylcyclohexione
Please help me calculate the undiluted samples ppm concentration.
My calculations were 280.11 ppm. Please see if I did my math correctly using the following standard curve.
Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EVSJL_W0qrxMkUjK2J3xMUEBHDu0UM1vPKQ-bc9HTcYXDQ?e=hVuPC4
Chapter 1 Solutions
Organic Chemistry
Ch. 1.3 - Give the ground-state electron configuration for...Ch. 1.3 - How many electrons does each of the following...Ch. 1.4 - Prob. 3PCh. 1.4 - Convert the following representation of ethane,...Ch. 1.4 - What are likely formulas for the following...Ch. 1.4 - Prob. 6PCh. 1.4 - Prob. 7PCh. 1.7 - Draw a line-bond structure for propane, CH3CH2CH3....Ch. 1.7 - Convert the following molecular model of hexane, a...Ch. 1.8 - Draw a line-bond structure for propene, CH3CH=CH2....
Ch. 1.8 - Draw a line-bond structure for 1, 3-butadiene,...Ch. 1.8 - Following is a molecular model of aspirin...Ch. 1.9 - Draw a line-bond structure for propyne, CH3C≡CH....Ch. 1.10 - Prob. 14PCh. 1.12 - Prob. 15PCh. 1.12 - Prob. 16PCh. 1.12 - The following molecular model is a representation...Ch. 1.SE - Convert each of the following molecular models...Ch. 1.SE - The following model is a representation of citric...Ch. 1.SE - The following model is a representation of...Ch. 1.SE - The following model is a representation of...Ch. 1.SE - How many valence electrons does each of the...Ch. 1.SE - Give the ground-state electron configuration for...Ch. 1.SE - Prob. 24APCh. 1.SE - Prob. 25APCh. 1.SE - Draw an electron-dot structure for acetonitrile,...Ch. 1.SE - Draw a line-bond structure for vinyl chloride,...Ch. 1.SE - Fill in any nonbonding valence electrons that are...Ch. 1.SE - Convert the following line-bond structures into...Ch. 1.SE - Convert the following molecular formulas into...Ch. 1.SE - Prob. 31APCh. 1.SE - Oxaloacetic acid, an important intermediate in...Ch. 1.SE - Prob. 33APCh. 1.SE - Potassium methoxide, KOCH3, contains both covalent...Ch. 1.SE - What is the hybridization of each carbon atom in...Ch. 1.SE - Prob. 36APCh. 1.SE - Prob. 37APCh. 1.SE - What bond angles do you expect for each of the...Ch. 1.SE - Propose structures for molecules that meet the...Ch. 1.SE - What kind of hybridization do you expect for each...Ch. 1.SE - Pyridoxal phosphate, a close relative of vitamin...Ch. 1.SE - Prob. 42APCh. 1.SE - Prob. 43APCh. 1.SE - Quetiapine, marketed as Seroquel, is a heavily...Ch. 1.SE - Tell the number of hydrogens bonded to each carbon...Ch. 1.SE - Why do you suppose no one has ever been able to...Ch. 1.SE - Allene, H2C=C=CH2, is somewhat unusual in that it...Ch. 1.SE - Allene (see Problem 1-47) is structurally related...Ch. 1.SE - Complete the electron-dot structure of caffeine,...Ch. 1.SE - Most stable organic species have tetravalent...Ch. 1.SE - A carbanion is a species that contains a...Ch. 1.SE - Divalent carbon species called carbenes are...Ch. 1.SE - There are two different substances with the...Ch. 1.SE - There are two different substances with the...Ch. 1.SE - There are two different substances with the...Ch. 1.SE - Prob. 56APCh. 1.SE - Among the most common over-the-counter drugs you...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide an IUPAC name for each of the compounds shown. (Specify (E)/(Z) stereochemistry, if relevant, for straight chain alkenes only. Pay attention to commas, dashes, etc.) H₁₂C C(CH3)3 C=C H3C CH3 CH3CH2CH CI CH3 Submit Answer Retry Entire Group 2 more group attempts remaining Previous Nextarrow_forwardArrange the following compounds / ions in increasing nucleophilicity (least to most nucleophilic) CH3NH2 CH3C=C: CH3COO 1 2 3 5 Multiple Choice 1 point 1, 2, 3 2, 1, 3 3, 1, 2 2, 3, 1 The other answers are not correct 0000arrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the cured electron-pushing arrows for thw following reaction or mechanistic steps. be sure to account for all bond-breaking and bond making stepsarrow_forward
- Using the graphs could you help me explain the answers. I assumed that both graphs are proportional to the inverse of time, I think. Could you please help me.arrow_forwardSynthesis of Dibenzalacetone [References] Draw structures for the carbonyl electrophile and enolate nucleophile that react to give the enone below. Question 1 1 pt Question 2 1 pt Question 3 1 pt H Question 4 1 pt Question 5 1 pt Question 6 1 pt Question 7 1pt Question 8 1 pt Progress: 7/8 items Que Feb 24 at You do not have to consider stereochemistry. . Draw the enolate ion in its carbanion form. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. ⚫ Separate multiple reactants using the + sign from the drop-down menu. ? 4arrow_forwardShown below is the mechanism presented for the formation of biasplatin in reference 1 from the Background and Experiment document. The amounts used of each reactant are shown. Either draw or describe a better alternative to this mechanism. (Note that the first step represents two steps combined and the proton loss is not even shown; fixing these is not the desired improvement.) (Hints: The first step is correct, the second step is not; and the amount of the anhydride is in large excess to serve a purpose.)arrow_forward
- Hi I need help on the question provided in the image.arrow_forwardDraw a reasonable mechanism for the following reaction:arrow_forwardDraw the mechanism for the following reaction: CH3 CH3 Et-OH Et Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. H± EXP. L CONT. י Α [1] осн CH3 а CH3 :Ö Et H 0 N о S 0 Br Et-ÖH | P LL Farrow_forward
- 20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward20.00 mL of 0.025 M HCl is titrated with 0.035 M KOH. What volume of KOH is needed?arrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY