
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1.SE, Problem 29AP
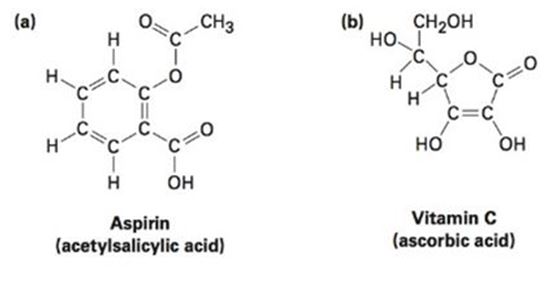
Convert the following line-bond structures into molecular formulas:
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
At the end of the silica gel production process, color changes occur during drying. Explain these color changes.
If CoCl2/H2O is dissolved in a mixture of H2O and concentrated HCl in a test tube, the tube is gently heated over a flame to approximately 80°C and then cooled externally. Explain the color changes that occur.
When producing silica gel, color changes occur at the end of the drying process. Explain these color changes.
Chapter 1 Solutions
Organic Chemistry
Ch. 1.3 - Give the ground-state electron configuration for...Ch. 1.3 - How many electrons does each of the following...Ch. 1.4 - Prob. 3PCh. 1.4 - Convert the following representation of ethane,...Ch. 1.4 - What are likely formulas for the following...Ch. 1.4 - Prob. 6PCh. 1.4 - Prob. 7PCh. 1.7 - Draw a line-bond structure for propane, CH3CH2CH3....Ch. 1.7 - Convert the following molecular model of hexane, a...Ch. 1.8 - Draw a line-bond structure for propene, CH3CH=CH2....
Ch. 1.8 - Draw a line-bond structure for 1, 3-butadiene,...Ch. 1.8 - Following is a molecular model of aspirin...Ch. 1.9 - Draw a line-bond structure for propyne, CH3C≡CH....Ch. 1.10 - Prob. 14PCh. 1.12 - Prob. 15PCh. 1.12 - Prob. 16PCh. 1.12 - The following molecular model is a representation...Ch. 1.SE - Convert each of the following molecular models...Ch. 1.SE - The following model is a representation of citric...Ch. 1.SE - The following model is a representation of...Ch. 1.SE - The following model is a representation of...Ch. 1.SE - How many valence electrons does each of the...Ch. 1.SE - Give the ground-state electron configuration for...Ch. 1.SE - Prob. 24APCh. 1.SE - Prob. 25APCh. 1.SE - Draw an electron-dot structure for acetonitrile,...Ch. 1.SE - Draw a line-bond structure for vinyl chloride,...Ch. 1.SE - Fill in any nonbonding valence electrons that are...Ch. 1.SE - Convert the following line-bond structures into...Ch. 1.SE - Convert the following molecular formulas into...Ch. 1.SE - Prob. 31APCh. 1.SE - Oxaloacetic acid, an important intermediate in...Ch. 1.SE - Prob. 33APCh. 1.SE - Potassium methoxide, KOCH3, contains both covalent...Ch. 1.SE - What is the hybridization of each carbon atom in...Ch. 1.SE - Prob. 36APCh. 1.SE - Prob. 37APCh. 1.SE - What bond angles do you expect for each of the...Ch. 1.SE - Propose structures for molecules that meet the...Ch. 1.SE - What kind of hybridization do you expect for each...Ch. 1.SE - Pyridoxal phosphate, a close relative of vitamin...Ch. 1.SE - Prob. 42APCh. 1.SE - Prob. 43APCh. 1.SE - Quetiapine, marketed as Seroquel, is a heavily...Ch. 1.SE - Tell the number of hydrogens bonded to each carbon...Ch. 1.SE - Why do you suppose no one has ever been able to...Ch. 1.SE - Allene, H2C=C=CH2, is somewhat unusual in that it...Ch. 1.SE - Allene (see Problem 1-47) is structurally related...Ch. 1.SE - Complete the electron-dot structure of caffeine,...Ch. 1.SE - Most stable organic species have tetravalent...Ch. 1.SE - A carbanion is a species that contains a...Ch. 1.SE - Divalent carbon species called carbenes are...Ch. 1.SE - There are two different substances with the...Ch. 1.SE - There are two different substances with the...Ch. 1.SE - There are two different substances with the...Ch. 1.SE - Prob. 56APCh. 1.SE - Among the most common over-the-counter drugs you...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Design experiments in UV-Vis to figure the optimal mole ratio of copper (1:1, 2:1, 3:1 and etc)versus ethambutol using all necessary chemicals including dihydrochloride and copper nitrate hemipentahydrate and sodium hydroxide. Show how UV-Vis absorbance and maximum wavelength would change in responsearrow_forwardCorrect each molecule in the drawing area below so that it has the condensed structure it would have if it were dissolv a 0.1 M aqueous solution of HCI. If there are no changes to be made, check the No changes box under the drawing area. No changes. HO—CH,—C—CH,—OH X 5 2 2 2 HO–CH,—CH,—C—CH,—OH Explanation Check Center Accessi ©2025 on 5 Carrow_forwardMake the calculations to prepare 2M H2SO4, from concentrated H2SO4 (98%; density: 1.84 g/mL).arrow_forward
- H CH3 CH3 b) Write the products of your compound and the following reagents. If the reaction would not work for your compound, write "no reaction" and explain the problem. NaCN H* H₂NNHCH5 H* -à NaBH -à CH2MgBr Cro₁₂ --à H3O+ -à c) Would your compound give a positive Tollen's test? Why or why not?arrow_forwardHomework 4 Chem 204 Dr. Hellwig Consider this compound, which will be referred to as "your compound". a) Name your compound according to the IUPAC system. Include stereochemistry (E/Z/R/S) H CH3 CH3arrow_forwardWhat is the mechanism for this?arrow_forward
- 21.50 Determine the combinations of haloalkane(s) and alkoxide(s) that could be used to synthesize the following ethers through Williamson ether synthesis. (a) (c) (d) (e) (f) H₂COarrow_forward1. Arrange the following in order of increasing bond energy (lowest bond energy first, highest bond energy last). Provide your rationale. C=C, C-F, C=C, C-N, C-C List the bond order for each example.arrow_forwardWhat is the major enolate formed when treated with LDA? And why that one?arrow_forward
- 4. Calculate the total number of sigma bonds and total number of pi bonds in each of the following compounds. a. HH :D: +1 I H-N-C-C-O-H I H b. HH H Н :N=C-C-C=C-CEC-H :0: total o H-C-H H-C = `C-H I H. 11 H-C = C= CH H total o total π total π 1 Harrow_forwardIn the following reaction, what quantity in moles of CH₃OH are required to give off 4111 kJ of heat? 2 CH₃OH (l) + 3 O₂ (g) → 2 CO₂ (g) + 4 H₂O(g) ∆H° = -1280. kJarrow_forwardIndicate the processes in the dismutation of Cu2O.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
INTRODUCTION TO MOLECULAR QUANTUM MECHANICS -Valence bond theory - 1; Author: AGK Chemistry;https://www.youtube.com/watch?v=U8kPBPqDIwM;License: Standard YouTube License, CC-BY