
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 1.SE, Problem 38AP
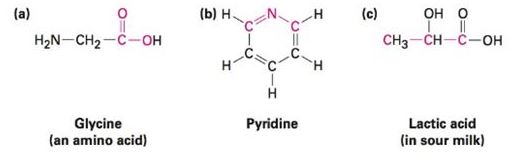
What bond angles do you expect for each of the following, and what kind of hybridization do you expect for the central atom in each?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
What is the final product when D-galactose reacts with hydroxylamine?
Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.
Chapter 1 Solutions
Organic Chemistry
Ch. 1.3 - Give the ground-state electron configuration for...Ch. 1.3 - How many electrons does each of the following...Ch. 1.4 - Prob. 3PCh. 1.4 - Convert the following representation of ethane,...Ch. 1.4 - What are likely formulas for the following...Ch. 1.4 - Prob. 6PCh. 1.4 - Prob. 7PCh. 1.7 - Draw a line-bond structure for propane, CH3CH2CH3....Ch. 1.7 - Convert the following molecular model of hexane, a...Ch. 1.8 - Draw a line-bond structure for propene, CH3CH=CH2....
Ch. 1.8 - Draw a line-bond structure for 1, 3-butadiene,...Ch. 1.8 - Following is a molecular model of aspirin...Ch. 1.9 - Draw a line-bond structure for propyne, CH3C≡CH....Ch. 1.10 - Prob. 14PCh. 1.12 - Prob. 15PCh. 1.12 - Prob. 16PCh. 1.12 - The following molecular model is a representation...Ch. 1.SE - Convert each of the following molecular models...Ch. 1.SE - The following model is a representation of citric...Ch. 1.SE - The following model is a representation of...Ch. 1.SE - The following model is a representation of...Ch. 1.SE - How many valence electrons does each of the...Ch. 1.SE - Give the ground-state electron configuration for...Ch. 1.SE - Prob. 24APCh. 1.SE - Prob. 25APCh. 1.SE - Draw an electron-dot structure for acetonitrile,...Ch. 1.SE - Draw a line-bond structure for vinyl chloride,...Ch. 1.SE - Fill in any nonbonding valence electrons that are...Ch. 1.SE - Convert the following line-bond structures into...Ch. 1.SE - Convert the following molecular formulas into...Ch. 1.SE - Prob. 31APCh. 1.SE - Oxaloacetic acid, an important intermediate in...Ch. 1.SE - Prob. 33APCh. 1.SE - Potassium methoxide, KOCH3, contains both covalent...Ch. 1.SE - What is the hybridization of each carbon atom in...Ch. 1.SE - Prob. 36APCh. 1.SE - Prob. 37APCh. 1.SE - What bond angles do you expect for each of the...Ch. 1.SE - Propose structures for molecules that meet the...Ch. 1.SE - What kind of hybridization do you expect for each...Ch. 1.SE - Pyridoxal phosphate, a close relative of vitamin...Ch. 1.SE - Prob. 42APCh. 1.SE - Prob. 43APCh. 1.SE - Quetiapine, marketed as Seroquel, is a heavily...Ch. 1.SE - Tell the number of hydrogens bonded to each carbon...Ch. 1.SE - Why do you suppose no one has ever been able to...Ch. 1.SE - Allene, H2C=C=CH2, is somewhat unusual in that it...Ch. 1.SE - Allene (see Problem 1-47) is structurally related...Ch. 1.SE - Complete the electron-dot structure of caffeine,...Ch. 1.SE - Most stable organic species have tetravalent...Ch. 1.SE - A carbanion is a species that contains a...Ch. 1.SE - Divalent carbon species called carbenes are...Ch. 1.SE - There are two different substances with the...Ch. 1.SE - There are two different substances with the...Ch. 1.SE - There are two different substances with the...Ch. 1.SE - Prob. 56APCh. 1.SE - Among the most common over-the-counter drugs you...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Linear Combination of Atomic Orbitals LCAO; Author: Edmerls;https://www.youtube.com/watch?v=nq1zwrAIr4c;License: Standard YouTube License, CC-BY
Quantum Molecular Orbital Theory (PChem Lecture: LCAO and gerade ungerade orbitals); Author: Prof Melko;https://www.youtube.com/watch?v=l59CGEstSGU;License: Standard YouTube License, CC-BY