
Interpretation:
For each of the following reaction the route that could reasonably be expected from the starting material to the product is to be provided.
Concept introduction:
Electrophiles are electron deficient species that have positive or partially positive charge. Lewis acids are electrophiles that accept electron pair.
Nucleophiles are electron rich species that have negative or partially negative charge. Lewis bases are nucleophiles that donate electron pair.
Free radical is an atom, molecule, or ion that has an unpaired electron, which makes it highly chemically reactive.
Substitution reaction: A reaction in which one of the hydrogen atoms of a hydrocarbon or a
Elimination reaction: A reaction in which two substituent groups are detached and a double bond is formed is called elimination reaction.
Addition reaction: It is the reaction in which unsaturated bonds are converted to saturated molecules by the addition of molecules.
The condensation reactions in carbonyl, the enolate or enol of a carbonyl group of one compound reacts with the carbonyl group of another compound. The two main types of condensation are Claisen and aldol condensation.
Claisen condensation entails the formation of 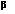 keto esters through carbon–carbon bond formation.
keto esters through carbon–carbon bond formation.
The Claisen condensation is the carbon–carbon bond formation reaction and is important for the preparation of
In Claisen condensation, the ester of one molecule is added to the carbonyl carbon of another molecule and the formation of
When one ester molecule has no alpha hydrogen, crossed Claisen condensation occurs.
Aldol condensation involves the reaction of enolate of an 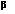 hydroxyl ketone or aldehyde.
hydroxyl ketone or aldehyde.
An aldol reaction takes place by acid catalysis, and direct dehydration of
A
A chemical reaction that is catalyzed by an base is called base catalyzed reaction.
Aldol reaction is preferred in basic condition over acidic condition as after the aldol condensation, acid catalysis promotes the reaction further.
A carbon–carbon bond formation occurs through aldol condensation reaction.
An aldol reaction takes place in a protic solvent with a base.
Dehydration of aldol addition product leads to the formation of conjugated
Trending nowThis is a popular solution!

Chapter 19 Solutions
Organic Chemistry
- What is the systematic name of the product P of this chemical reaction? 010 HO-CH2-CH2-C-OH ☐ + NaOH P+ H2Oarrow_forward1. Provide missing starting materials, reagents, products. If a product cannot be made, write NP (not possible) in the starting material box. a) C10H12 Ph OMe AcOHg+ + enantiomer Br C6H10O2 + enantiomerarrow_forwardDraw the Fischer projection of the most common naturally-occurring form of cysteine, with the acid group at the top and the side chain at the bottom. Important: be sure your structure shows the molecule as it would exist at physiological pH. Click and drag to start drawing a structure. :☐ ©arrow_forward
- Draw an a amino acid with an ethyl (-CH2-CH3) side chain. Draw the molecule as it would appear at physiological pH. Click and drag to start drawing a structure. :□ S टेarrow_forwardWrite the systematic name of each organic molecule: HO Cl structure O OH O HO OH name ☐ OH OH ☐ OH ☐arrow_forwardWrite the name of a naturally-occuring hydrophillic amino acid. (You will find the structures of the naturally-occuring amino acids in the ALEKS Data resource.) × $arrow_forward
- Please note that it is correct and explains it rightly:The proportion of O, C and H in the graphite oxide is:a) Constant, for the quantities of functional groups of acids, phenols, epoxy, etc. its constantsb) Depending on the preparation method, as much oxidant as the graphite is destroyed and it has less oxygenc) Depends on the structure of the graphic being processed, whether it can be more three-dimensional or with larger crystals, or with smaller crystals and more borders.arrow_forwardThe proportion of O, C and H in the graphite oxide is constant, only the cantidades of functional groups of acids, phenols, epoxy, etc. its constants. ¿Is it correct?arrow_forwardThe proportion of O, C and H in the graphite oxide depends on the structure of the graph that is processed, which may be more tridimensional or with larger crystals, or with smaller crystals and more borders. ¿Is it correct?arrow_forward
- In mixed oxides with superconducting properties, we find Cu:a) Frequentlyb) Alwaysc) Almost neverarrow_forwardThe proportion of O, C and H in the graphite oxide depends on the preparation method, as long as the most oxidant, the most graphite is destroyed and has less O. Is it correct?arrow_forwardWrite the complete common (not IUPAC) name of each molecule below. Note: if a molecule is one of a pair of enantiomers, be sure you start its name with D- or L- so we know which enantiomer it is. molecule C=O H3N CH3 common name (not the IUPAC name) H ☐ C=O H O-C-CH2-CH2 010 NH3 ☐ H3N ☐ HO 5arrow_forward
