
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19, Problem 56P
Allowing acetone to react with 2 molar equivalents of benzaldehyde in the presence of KOH in ethanol leads to the formation of compound X. The
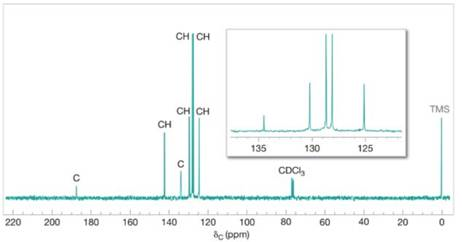
FIGURE 19.1 The broadband proton-decoupled
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.
Write the amididation reaction mechanism of p-aminophenol and acetic acid to produce acetaminophen please use arrows.
Name the following using IUPAC.
Chapter 19 Solutions
Organic Chemistry
Ch. 19 - PRACTICE PROBLEM 19.1 (a) Write a mechanism. for...Ch. 19 - Practice Problem 19.2
Since the products obtained...Ch. 19 - Prob. 3PPCh. 19 - PRACTICE PROBLEM 19.4
Write mechanisms that...Ch. 19 - Prob. 5PPCh. 19 - Prob. 6PPCh. 19 - Practice Problem 19.7 The acid-catalyzed aldol...Ch. 19 - Prob. 8PPCh. 19 - Practice Problem 19.9
(a) Provide a mechanism for...Ch. 19 - Prob. 10PP
Ch. 19 - Practice Problem 19.11 Outlined below is a...Ch. 19 - Prob. 12PPCh. 19 - Prob. 13PPCh. 19 - Prob. 14PPCh. 19 - Practice Problem 19.15
Starting with ketones and...Ch. 19 - Practice Problem 19.16 Assuming that dehydration...Ch. 19 - Practice Problem 19.17 What starting compound...Ch. 19 - Practice Problem 19.18
What experimental...Ch. 19 - Prob. 19PPCh. 19 - Practice Problem 19.20
When acrolein (propenal)...Ch. 19 - Prob. 21PPCh. 19 - PRACTICE PROBLEM 19.22
Qutline reasonable...Ch. 19 - Prob. 23PCh. 19 - Show all steps in the following syntheses. You may...Ch. 19 - Prob. 25PCh. 19 - Prob. 26PCh. 19 - Prob. 27PCh. 19 - 19.28 Show how the diketone at the right could be...Ch. 19 - Prob. 29PCh. 19 - 19.30 Write a detailed mechanism for the following...Ch. 19 - Prob. 31PCh. 19 - Prob. 32PCh. 19 - 19.33 Predict the products from each of the...Ch. 19 - Prob. 34PCh. 19 - Show how each of the following transformations...Ch. 19 - Prob. 36PCh. 19 - What reagents would you use to bring about each...Ch. 19 - Prob. 38PCh. 19 - Prob. 39PCh. 19 - 19.40 When the aldol reaction of acetaldehyde is...Ch. 19 - Prob. 41PCh. 19 - Prob. 42PCh. 19 - 19.43 The following reaction illustrates the...Ch. 19 - What is the structure of the cyclic compound that...Ch. 19 - Prob. 45PCh. 19 - Prob. 46PCh. 19 - Prob. 47PCh. 19 - Predict the products from the following reactions....Ch. 19 - Prob. 49PCh. 19 - Prob. 50PCh. 19 - Prob. 51PCh. 19 - Prob. 52PCh. 19 - Prob. 53PCh. 19 - The Perkin condensation is an aldol-type...Ch. 19 - 19.55 (a) Infrared spectroscopy provides an easy...Ch. 19 - Allowing acetone to react with 2 molar equivalents...Ch. 19 - (+) Fenchone is a terpenoid that can be isolated...Ch. 19 - Prob. 58PCh. 19 - Prob. 59PCh. 19 - 19.60 Develop a synthesis for the following...Ch. 19 - 19.61 Provide a mechanism for each of the...Ch. 19 - 19.62 (a) Deduce the structure of product A,...Ch. 19 - Prob. 63PCh. 19 - Prob. 1LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
How does an obligate aerobe differ from a facultative aerobe?
Brock Biology of Microorganisms (15th Edition)
2. In uniform circular motion, which of the following quantities are constant: speed, instantaneous velocity, c...
College Physics: A Strategic Approach (3rd Edition)
1. Which parts of the skeleton belong to the appendicular skeleton? Which belong to the axial skeleton?
Human Anatomy & Physiology (2nd Edition)
A wild-type fruit fly (heterozygous for gray body color and led eyes) is mated Willi a black fruit fly wltli pu...
Campbell Biology (11th Edition)
90. Classify each chemical reaction as a synthesis, decomposition, single-displacement, or double-displacement ...
Introductory Chemistry (6th Edition)
1.6 Read the labels on products used to wash your dishes. What are the names of some chemicals contained in tho...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forwardName the following molecules with IUpacarrow_forward
- What is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forward
- Please help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forwardProvide solutionsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY