
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19, Problem 28P
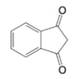
Show how the diketone at the right could be prepared by a condensation reaction:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Topics]
[References]
Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.)
Keep the information page open for feedback reference.
H
The IUPAC name is
[Review Topics]
[References]
Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.)
Keep the information page open for feedback reference.
The IUPAC name is
Submit Answer
Retry Entire Group
9 more group attempts remaining
Please draw.
Chapter 19 Solutions
Organic Chemistry
Ch. 19 - PRACTICE PROBLEM 19.1 (a) Write a mechanism. for...Ch. 19 - Practice Problem 19.2
Since the products obtained...Ch. 19 - Prob. 3PPCh. 19 - PRACTICE PROBLEM 19.4
Write mechanisms that...Ch. 19 - Prob. 5PPCh. 19 - Prob. 6PPCh. 19 - Practice Problem 19.7 The acid-catalyzed aldol...Ch. 19 - Prob. 8PPCh. 19 - Practice Problem 19.9
(a) Provide a mechanism for...Ch. 19 - Prob. 10PP
Ch. 19 - Practice Problem 19.11 Outlined below is a...Ch. 19 - Prob. 12PPCh. 19 - Prob. 13PPCh. 19 - Prob. 14PPCh. 19 - Practice Problem 19.15
Starting with ketones and...Ch. 19 - Practice Problem 19.16 Assuming that dehydration...Ch. 19 - Practice Problem 19.17 What starting compound...Ch. 19 - Practice Problem 19.18
What experimental...Ch. 19 - Prob. 19PPCh. 19 - Practice Problem 19.20
When acrolein (propenal)...Ch. 19 - Prob. 21PPCh. 19 - PRACTICE PROBLEM 19.22
Qutline reasonable...Ch. 19 - Prob. 23PCh. 19 - Show all steps in the following syntheses. You may...Ch. 19 - Prob. 25PCh. 19 - Prob. 26PCh. 19 - Prob. 27PCh. 19 - 19.28 Show how the diketone at the right could be...Ch. 19 - Prob. 29PCh. 19 - 19.30 Write a detailed mechanism for the following...Ch. 19 - Prob. 31PCh. 19 - Prob. 32PCh. 19 - 19.33 Predict the products from each of the...Ch. 19 - Prob. 34PCh. 19 - Show how each of the following transformations...Ch. 19 - Prob. 36PCh. 19 - What reagents would you use to bring about each...Ch. 19 - Prob. 38PCh. 19 - Prob. 39PCh. 19 - 19.40 When the aldol reaction of acetaldehyde is...Ch. 19 - Prob. 41PCh. 19 - Prob. 42PCh. 19 - 19.43 The following reaction illustrates the...Ch. 19 - What is the structure of the cyclic compound that...Ch. 19 - Prob. 45PCh. 19 - Prob. 46PCh. 19 - Prob. 47PCh. 19 - Predict the products from the following reactions....Ch. 19 - Prob. 49PCh. 19 - Prob. 50PCh. 19 - Prob. 51PCh. 19 - Prob. 52PCh. 19 - Prob. 53PCh. 19 - The Perkin condensation is an aldol-type...Ch. 19 - 19.55 (a) Infrared spectroscopy provides an easy...Ch. 19 - Allowing acetone to react with 2 molar equivalents...Ch. 19 - (+) Fenchone is a terpenoid that can be isolated...Ch. 19 - Prob. 58PCh. 19 - Prob. 59PCh. 19 - 19.60 Develop a synthesis for the following...Ch. 19 - 19.61 Provide a mechanism for each of the...Ch. 19 - 19.62 (a) Deduce the structure of product A,...Ch. 19 - Prob. 63PCh. 19 - Prob. 1LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
7.42(a) What is the trend in first ionization energies as one proceeds down the group 7A elements? Explain ho...
Chemistry: The Central Science (14th Edition)
What are the general developmental trends during the fetal period?
Principles of Anatomy and Physiology
8. A human maintaining a vegan diet (containing no animal products) would be a:
a. producer
b. primary consume...
Human Biology: Concepts and Current Issues (8th Edition)
Flask A contains yeast cells in glucose-minimal salts broth incubated at 30C with aeration. Flask B contains ye...
Microbiology: An Introduction
What two components contribute to species diversity? Explain how two communities with the same number of specie...
Campbell Biology (11th Edition)
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chromatogram with ideal Gaussian bands has tR = 9.0 minutes and w1/2 = 2.0 minutes. Find the number of theoretical plates that are present, and calculate the height of each theoretical plate if the column is 10 centimeters long.arrow_forwardAn open tubular column has an inner diameter of 207 micrometers, and the thickness of the stationary phase on the inner wall is 0.50 micrometers. Unretained solute passes through in 63 seconds and a particular solute emerges at 433 seconds. Find the distribution constant for this solute and find the fraction of time spent in the stationary phase.arrow_forwardConsider a chromatography column in which Vs= Vm/5. Find the retention factor if Kd= 3 and Kd= 30.arrow_forward
- To improve chromatographic separation, you must: Increase the number of theoretical plates on the column. Increase the height of theoretical plates on the column. Increase both the number and height of theoretical plates on the column. Increasing the flow rate of the mobile phase would Increase longitudinal diffusion Increase broadening due to mass transfer Increase broadening due to multiple paths You can improve the separation of components in gas chromatography by: Rasing the temperature of the injection port Rasing the temperature of the column isothermally Rasing the temperature of the column using temperature programming In GC, separation between two different solutes occurs because the solutes have different solubilities in the mobile phase the solutes volatilize at different rates in the injector the solutes spend different amounts of time in the stationary phasearrow_forwardplease draw and example of the following: Show the base pair connection(hydrogen bond) in DNA and RNAarrow_forwardNaming and drawing secondary Write the systematic (IUPAC) name for each of the following organic molecules: CH3 Z structure CH3 CH2 CH2 N-CH3 CH3-CH2-CH2-CH-CH3 NH CH3-CH-CH2-CH2-CH2-CH2-CH2-CH3 Explanation Check ☐ name ☐ 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C Garrow_forward
- C This question shows how molecular orbital (MO) theory can be used to understand the chemical properties of elemental oxygen O₂ and its anionic derivative superoxide Oz. a) Draw the MO energy diagram for both O2 and O2. Clearly label your diagram with atomic orbital names and molecular orbital symmetry labels and include electrons. Draw the Lewis structure of O2. How does the MO description of O2 differ from the Lewis structure, and how does this difference relate to the high reactivity and magnetic properties of oxygen? ) Use the MO diagram in (a) to explain the difference in bond length and bond energy between superoxide ion (Oz, 135 pm, 360 kJ/mol) and oxygen (O2, 120.8 pm, 494 kJ/mol).arrow_forwardPlease drawarrow_forward-Page: 8 nsition metal ions have high-spin aqua complexes except one: [Co(HO)₁]". What is the d-configuration, oxidation state of the metal in [Co(H:O))"? Name and draw the geometry of [Co(H2O)]? b) Draw energy diagrams showing the splitting of the five d orbitals of Co for the two possible electron configurations of [Co(H2O)]: Knowing that A = 16 750 cm and Пl. = 21 000 cm, calculate the configuration energy (.e., balance or ligand-field stabilization energy and pairing energy) for both low spin and high spin configurations of [Co(H2O)]. Which configuration seems more stable at this point of the analysis? (Note that 349.76 cm = 1 kJ/mol) Exchange energy (IT) was not taken into account in part (d), but it plays a role. Assuming exchange an occur within t29 and within eg (but not between tz, and ea), how many exchanges are possible in the low in configuration vs in the high spin configuration? What can you say about the importance of exchange energy 07arrow_forward
- Draw everything please on a piece of paper explaining each steparrow_forwardDefine crystalline, polycrystalline and amorphous materials What crystal system and Bravais lattices are shown in the figure immediately below? What do a, b, C, a, ẞ and y represent and what are their values? You can label the Bravais lattices directly above or under the figure. C aarrow_forward32. The diagrams below show the band structure of an intrinsic semiconductor at absolute zero and room temperature. Room Temperature EF E OK Ep- a) In the space below, sketch a similar pair of diagrams for an n-type semiconductor. D) Give the definition and an example of (i) an intrinsic semiconductor and (ii) an n-type semiconductor.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License