
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 19, Problem 19.7P
Interpretation Introduction
(a)
Interpretation:
The products formed when ethyl benzoate is treated with the following set of reagents should be determined.
H2 O, NaOH, heat.
Concept Introduction:
Base hydrolysis of an ester produces sodium salt of the
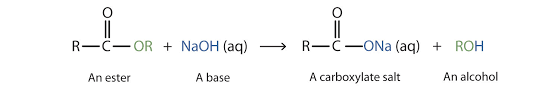
Interpretation Introduction
(b)
Interpretation:
The products formed when ethyl benzoate is treated with the following set of reagents should be determined.
H2 O, HCl, heat.
Concept Introduction:
Acid hydrolysis of an ester produces carboxylic acid and alcohol.
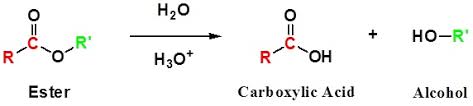
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Predict the organic products that form in the reaction below:
OH
H+
H+
+
☑
Y
Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the products.
In the drawing area below, draw the skeletal ("line") structures of the missing organic products X and Y. You may draw the structures in any arrangement that
you like, so long as they aren't touching.
Click and drag to start drawing a
structure.
✓
m
Determine the structures of the missing organic molecules in the following reaction:
+ H₂O
+H
H+
Y
Z
☑
☑
Note: Molecules that share the same letter have the exact same structure.
In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X, Y, and Z. You may draw the structures in any arrangement
that you like, so long as they aren't touching. Molecule X shows up in multiple steps, but you only have to draw its structure once.
Click and drag to start drawing a structure.
AP
+
Please help, this is all the calculations
i got!!! I will rate!!!Approx mass of
KMnO in vial: 3.464
4
Moss of beaker 3×~0. z Nax200:
= 29.9219
Massof weacerv after remosimgain
N2C2O4. Need to fill in all the
missing blanks.
ง
ง
Approx mass of KMnO4 in vials 3.464
Mass of beaker + 3x ~0-304: 29.9219
2~0.20
Miss of beaker + 2x-
29.7239
Mass of beaker + 1x~0.2g Naz (204
29-5249
Mass of beaver after removing as
qa Na₂ C₂O
T1
T2
T3
Final Buiet reading
Initial butet reading (int))
Hass of NaOr used for Titration
-reading (mL)
calculation Results:
8.5ml
17mL
27.4mL
Oml
Om
Oml
T1
T2
T3
Moles of No CO
Moles of KMO used
LOF KM. O used
Molenty of KMNO
Averagem Of KMOWL
Chapter 19 Solutions
Introduction to General, Organic and Biochemistry
Ch. 19.1 - Prob. 19.1PCh. 19.4 - Problem 19-2 Complete the equation for each...Ch. 19.4 - Prob. 19.3PCh. 19 - Prob. 19.4PCh. 19 - Write the IUPAC name for each compound.Ch. 19 - Prob. 19.6PCh. 19 - Prob. 19.7PCh. 19 - Prob. 19.8PCh. 19 - Prob. 19.9PCh. 19 - 0 Complete the equations for these reactions.
Ch. 19 - Prob. 19.11PCh. 19 - Prob. 19.12PCh. 19 - Prob. 19.13PCh. 19 - Prob. 19.14PCh. 19 - Prob. 19.15PCh. 19 - 6 Why are Dacron and Mylar referred to as...Ch. 19 - 7 What type of structural feature do the...Ch. 19 - Prob. 19.18PCh. 19 - Prob. 19.19PCh. 19 - 0 Show how triphosphoric acid can form from three...Ch. 19 - 1 Write an equation for the hydrolysis of...Ch. 19 - 2 (Chemical Connections 19A) Locate the ester...Ch. 19 - Prob. 19.23PCh. 19 - Prob. 19.24PCh. 19 - Prob. 19.25PCh. 19 - Prob. 19.26PCh. 19 - Prob. 19.27PCh. 19 - 8 (Chemical Connections 19C) Once it has been...Ch. 19 - Prob. 19.29PCh. 19 - Prob. 19.30PCh. 19 - Prob. 19.31PCh. 19 - Prob. 19.32PCh. 19 - Prob. 19.33PCh. 19 - 4 (Chemical Connections 19F) Why do Lactomer...Ch. 19 - Prob. 19.35PCh. 19 - Prob. 19.36PCh. 19 - Prob. 19.37PCh. 19 - 8 In Chapter 22, we will discuss a class of...Ch. 19 - Prob. 19.39PCh. 19 - Prob. 19.40PCh. 19 - Prob. 19.41PCh. 19 - Prob. 19.42PCh. 19 - Prob. 19.43PCh. 19 - Prob. 19.44PCh. 19 - Prob. 19.45PCh. 19 - Prob. 19.46PCh. 19 - Prob. 19.47PCh. 19 - Prob. 19.48PCh. 19 - Prob. 19.49P
Knowledge Booster
Similar questions
- Draw the skeletal ("line") structure of 2-hydroxy-4-methylpentanal. Click and drag to start drawing a structure. Xarrow_forwardDetermine whether the following molecule is a hemiacetal, acetal, or neither and select the appropriate box below. Also, highlight the hemiacetal or acetal carbon if there is one. hemiacetal acetal Oneither OHarrow_forwardWhat is the missing reactant R in this organic reaction? ་ ་ ་ ་ ་ ་ ་ ་ ་ ་ +R H3O+ • Draw the structure of R in the drawing area below. N • Be sure to use wedge and dash bonds if it's necessary to draw one particular enantiomer. Click and drag to start drawing a structure.arrow_forward
- Write the systematic name of each organic molecule: H structure H OH OH H OH name ☐ OHarrow_forwardDetermine whether each of the following molecules is a hemiacetal, acetal, or neither and select the appropriate box in the table. CH3O OH OH OH hemiacetal acetal neither hemiacetal acetal neither Xarrow_forwardWhat is the missing reactant R in this organic reaction? N N དལ་ད་་ + R • Draw the structure of R in the drawing area below. • Be sure to use wedge and dash bonds if it's necessary to draw one particular enantiomer. Click and drag to start drawing a structure. ㄖˋarrow_forward
- Draw the condensed structure of 4-hydroxy-3-methylbutanal. Click anywhere to draw the first atom of your structure.arrow_forwardUsing the bond energy values, calculate the energy that must be supplied or is released upon the polymerization of 755 monomers. If energy must be supplied, provide a positive number; if energy is released, provide a negative number. Hint: Avogadro’s number is 6.02 × 1023.arrow_forward-AG|F=2E|V 3. Before proceeding with this problem you may want to glance at p. 466 of your textbook where various oxo-phosphorus derivatives and their oxidation states are summarized. Shown below are Latimer diagrams for phosphorus at pH values at 0 and 14: Acidic solution -0.93 +0.38 -0.51 -0.06 H3PO4 →H4P206 H3PO3 H3PO2 → P→ PH3 -0.28 -0.50 → -0.50 Basic solution 3-1.12 -1.57 -2.05 -0.89 PO HPO →→H2PO2 P PH3 -1.73 a) Under acidic conditions, H3PO4 can be reduced into H3PO3 directly (-0.28V), or via the formation and reduction of H4P2O6 (-0.93/+0.38V). Calculate the values of AG's for both processes; comment. (3 points) 0.5 PH, 0.0 -0.5- 2 3 9 3 -1.5 -2.0 Pa H,PO H,PO H,PO -3 -1 0 2 4 Oxidation state, N 2 b) Frost diagram for phosphorus under acidic conditions is shown. Identify possible disproportionation and comproportionation processes; write out chemical equations describing them. (2 points) c) Elemental phosphorus tends to disproportionate under basic conditions. Use data in…arrow_forward
- These two reactions appear to start with the same starting materials but result in different products. How do the chemicals know which product to form? Are both products formed, or is there some information missing that will direct them a particular way?arrow_forwardWhat would be the best choices for the missing reagents 1 and 3 in this synthesis? 1. PPh3 3 1 2 2. n-BuLi • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Priva ×arrow_forwardPredict the products of this organic reaction: Explanation Check IN NaBH3CN H+ ? Click and drag to start drawing a structure. D 5 C +arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning