
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 19, Problem 19.15P
Interpretation Introduction
Interpretation:
To draw short sections of two parallel chains of nylon-66(each running in the same direction) and if it is possible to align them in such a way that there is hydrogen bonding between the N-H groups of one chain and the C=O groups of the parallel chain should be explained.
Concept Introduction:
A hydrogen bond is formed by a partial electrostatic attraction between hydrogen (H) atom attached to an electronegative atom (nitrogen (N), oxygen (O), or fluorine (F)) and other electronegative atom and lone pair present in the vicinity of it.
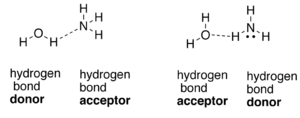
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Identify priority of the substituents:
CH3
How many chiral carbons are in the molecule?
OH
F
CI
Br
A mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC
with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots
of R, 0.1 and 0.2 and 0.3.
Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest
(Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively?
0
CH:
0
CH,
0
H.C
OH
H.CN
OH
Acet-B
Rin-C
phen-A
A
A
<
Chapter 19 Solutions
Introduction to General, Organic and Biochemistry
Ch. 19.1 - Prob. 19.1PCh. 19.4 - Problem 19-2 Complete the equation for each...Ch. 19.4 - Prob. 19.3PCh. 19 - Prob. 19.4PCh. 19 - Write the IUPAC name for each compound.Ch. 19 - Prob. 19.6PCh. 19 - Prob. 19.7PCh. 19 - Prob. 19.8PCh. 19 - Prob. 19.9PCh. 19 - 0 Complete the equations for these reactions.
Ch. 19 - Prob. 19.11PCh. 19 - Prob. 19.12PCh. 19 - Prob. 19.13PCh. 19 - Prob. 19.14PCh. 19 - Prob. 19.15PCh. 19 - 6 Why are Dacron and Mylar referred to as...Ch. 19 - 7 What type of structural feature do the...Ch. 19 - Prob. 19.18PCh. 19 - Prob. 19.19PCh. 19 - 0 Show how triphosphoric acid can form from three...Ch. 19 - 1 Write an equation for the hydrolysis of...Ch. 19 - 2 (Chemical Connections 19A) Locate the ester...Ch. 19 - Prob. 19.23PCh. 19 - Prob. 19.24PCh. 19 - Prob. 19.25PCh. 19 - Prob. 19.26PCh. 19 - Prob. 19.27PCh. 19 - 8 (Chemical Connections 19C) Once it has been...Ch. 19 - Prob. 19.29PCh. 19 - Prob. 19.30PCh. 19 - Prob. 19.31PCh. 19 - Prob. 19.32PCh. 19 - Prob. 19.33PCh. 19 - 4 (Chemical Connections 19F) Why do Lactomer...Ch. 19 - Prob. 19.35PCh. 19 - Prob. 19.36PCh. 19 - Prob. 19.37PCh. 19 - 8 In Chapter 22, we will discuss a class of...Ch. 19 - Prob. 19.39PCh. 19 - Prob. 19.40PCh. 19 - Prob. 19.41PCh. 19 - Prob. 19.42PCh. 19 - Prob. 19.43PCh. 19 - Prob. 19.44PCh. 19 - Prob. 19.45PCh. 19 - Prob. 19.46PCh. 19 - Prob. 19.47PCh. 19 - Prob. 19.48PCh. 19 - Prob. 19.49P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I need the nomenclature of this compoundarrow_forward2. Name the following hydrocarbons. (9 marks) a) HHHHHHHH H-C-C- H-O-S b) HCEC-CH3 H H H H H d) c) H C=C- H H H e) CH3 CH3 CH2CH=CH-CH=CHCH3 HHHH H-C-C-C-C-H H HH H f) large CH2CH3 pola H3C section lovels tower, able ocart firs g) Tower H3C-CH2 then in H3C-CH-CH-CH3 enblbano bne noitsidab Copyright © 2008. Durham Continuing Education CH3arrow_forwardName the molecules & Identify any chiral center CH3CH2CH2CHCH₂CH₂CH₂CH₂ OH CH₂CHCH2CH3 Br CH3 CH3CHCH2CHCH2CH3 CH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY