
Concept explainers
(a)
Interpretation:
The molecular formula of peramivir should be determined.
Concept Introduction:
Peramivir is an antiviral drug which is used in the treatment of influenza. It is a neuraminidase inhibitor which inhibits the transition state of the influenza so that it does not produce new viruses from the infected cells.
Molecular formula of a molecule represents the total number of atoms present in the molecule.
Answer to Problem 19.46P
C15 H28 N4 O4.
Explanation of Solution
The structure of the molecule is as follows:
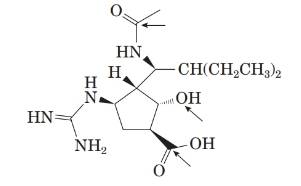
From the structure, the molecular formula of the compound is C15 H28 N4 O4.
(b)
Interpretation:
The
Concept Introduction:
Functional group of a compound represents its chemical properties. The most common organic functional groups are alcohol,
In amines, the alkyl group is attached to the nitrogen atom having 2 hydrogen atoms and one lone pair on it.
Depending on the number of alkyl groups attached to nitrogen atom, they are classified as primary (1 alkyl group), secondary (2 alkyl groups) and tertiary (3 alkyl groups).
In alcohols, the alkyl group is attached to a hydroxyl group. Similar to amines, depending on the number of alkyl groups they are classified as primary, secondary and tertiary.
Carboxylic acid, ketone and aldehyde have common carbonyl group which is attached to hydroxyl group in carboxylic group, hydrogen atom in aldehyde and two alkyl groups in ketone.
Explanation of Solution
The structure of the molecule is as follows:
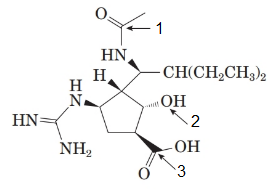
The labelled groups are as follows:
- Amide
- Alcohol
- Carboxylic acid
Here, nitrogen in the amide group and alcohol (hydroxyl group) both are secondary.
(c)
Interpretation:
The number of stereocenters present in peramivir should be determined.
Concept Introduction:
Stereocentre or chiral centre is the carbon centre when four different groups attached to it. For a molecule with n stereocenters, the number of stereoisomers can be calculated using the following formula:
Answer to Problem 19.46P
5 stereocenters.
Explanation of Solution
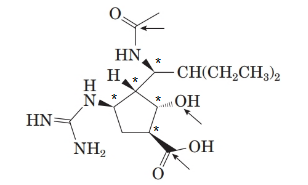
The structure of the molecule is as follows:
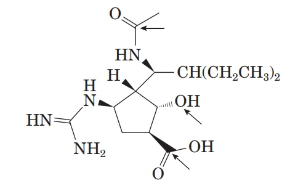
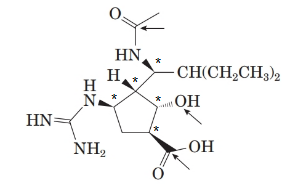
Stereocentre or chiral centre is the carbon centre when four different groups attached to it. All the stereocenters present in the compound are represented as follows:
(d)
Interpretation:
The maximum number of stereoisomers should be calculated.
Concept Introduction:
Stereocentre or chiral centre is the carbon centre when four different groups attached to it. For a molecule with n stereocenters, the number of stereoisomers can be calculated using the following formula:
Answer to Problem 19.46P
32 stereoisomers.
Explanation of Solution
The number of stereocenters in the compound is 5.
Thus, maximum number of stereoisomers can be calculated as follows:
Putting the value,
Thus, there are 32 stereoisomers in the molecule.
(e)
Interpretation:
The number of enantiomers and diastereomers of peramivir should be determined.
Concept Introduction:
Enantiomers are two stereoisomers which are non-superimposable mirror image of each other. Two enantiomers of a compound have similar physical properties but the direction of rotation of polarized light is different. The interaction with the optical isomers of other compounds is also different for them.
Due to this, there are different biological effects of different enantiomers of a compound. Optical activity can be observed in pure enantiomers. They can be separated making use of a chiral agent.
Naturally, there is only one enantiomer possible for most of the chiral compounds which are biologically present. For example, glycine in amino acids.
Diastereomer.
They are stereoisomers which are not mirror image of each other. The following isomers comes under this category:
Meso compounds, E-Z isomers, cis-trans isomers etc.
They also have similar physical properties.
One should not confuse with the D- and L- notation of the isomers with commonly used d- and l- labelling.
(e)
Answer to Problem 19.46P
There are 12 enantiomers and 30 diastereomers of peramivir.
Explanation of Solution
For any molecule, number of enantiomers and diastereomers can be calculated as follows:
For even number of stereocenters, the number of enantiomers can be calculated as follows:
And, for odd number of stereocenters, the number of enantiomers can be calculated as follows:
Since, the number of stereocenters are 5, it follows the formula for odd.
Putting the value,
Thus, number of enantiomers will be 12.
Now, now of diastereomers can be calculated as follows:
Putting the value,
Thus, number of diastereomers will be 30.
(f)
Interpretation:
The stereocenter with R-Configuration should be identified.
Concept Introduction:
Peramivir is an antiviral drug which is used in the treatment of influenza. It is a neuraminidase inhibitor which inhibits the transition state of the influenza so that it does not produce new viruses from the infected cells.
Answer to Problem 19.46P
The stereocenter with R-configuration is represented as follows:
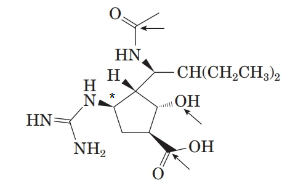
Explanation of Solution
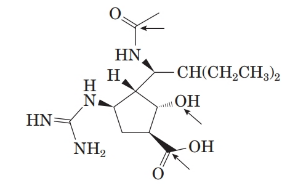
The structure of molecule is as follows:
There are 5 stereoisomers in the above molecule labelled as follows:
To name the molecule, the priory order for the atoms in ring will be:
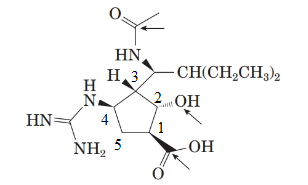
This is done by considering the functional group priority. Carboxylic acid gets the priority order one.
From the spectral data, chemical name of peramivir is ([1S, 2S, 3S, 4R]-3-[(1S)-1-(acetylamino)-2-ethylbutyl]-4-[(aminoiminomethyl)amino]-2-hydroxy cyclopentane carboxylic acid. Thus, the stereocenter at 4th position in the 5 membered ring has the r configuration.
Therefore, the stereocenter with R-configuration is represented as follows:
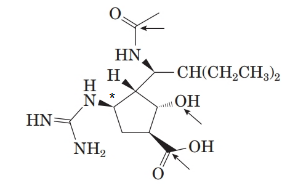
(g)
Interpretation:
The given molecules should be classified as identical, enantiomer, diastereomer and none of these.
Concept Introduction:
Enantiomers are two stereoisomers which are non-superimposable mirror image of each other. Two enantiomers of a compound have similar physical properties but the direction of rotation of polarized light is different. The interaction with the optical isomers of other compounds is also different for them.
Due to this, there are different biological effects of different enantiomers of a compound. Optical activity can be observed in pure enantiomers. They can be separated making use of a chiral agent.
Naturally, there is only one enantiomer possible for most of the chiral compounds which are biologically present. For example, glycine in amino acids.
Diastereomer.
They are stereoisomers which are not mirror image of each other. The following isomers comes under this category:
Meso compounds, E-Z isomers, cis-trans isomers etc.
They also have similar physical properties.
One should not confuse with the D- and L- notation of the isomers with commonly used d- and l- labelling.
Answer to Problem 19.46P
A- Identical.
B- Diastereomers.
C- Diastereomers.
D-Enantiomers.
Explanation of Solution
The structure of the peramivir is as follows:
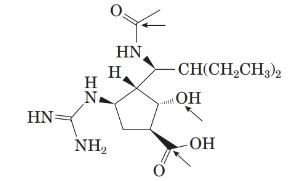
Comparing the molecular structure, A with the given structure of peramivir as follows:
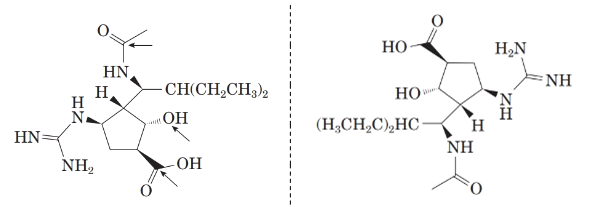
Both are not mirror image of each other. They are identical to each other. The second structure on the right-hand side formed by 180-degree rotation of the molecule given in left.
They are neither diastereomers nor enantiomers.
Now, comparing the molecular structure B with the given structure of peramivir as follows:
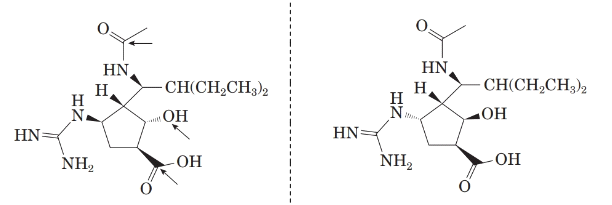
The molecules are not mirror image of each other. Thus, they are diastereomers.
Now, comparing the molecular structure C with the given structure of peramivir as follows:
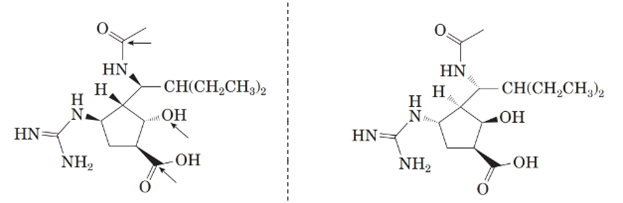
They are not mirror image of each other. The molecules are diastereomers.
Now, comparing the molecular structure D with the given structure of peramivir as follows:
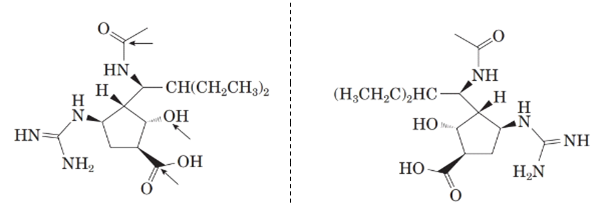
From the above figure, both the structures are mirror image of each other. Also, the mirror images are non-superimposable. Thus, they are enantiomers of each other.
Want to see more full solutions like this?
Chapter 19 Solutions
Introduction to General, Organic and Biochemistry
- Draw the complete mechanism for the acid-catalyzed hydration of this alkene. esc 田 Explanation Check 1 888 Q A slock Add/Remove step Q F4 F5 F6 A བྲA F7 $ % 5 @ 4 2 3 & 6 87 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce W E R T Y U S D LL G H IK DD 요 F8 F9 F10 F1 * ( 8 9 0 O P J K L Z X C V B N M H He commandarrow_forwardExplanation Check F1 H₂O H₂ Pd 1) MCPBA 2) H3O+ 1) Hg(OAc)2, H₂O 2) NaBH4 OH CI OH OH OH hydration halohydrin formation addition halogenation hydrogenation inhalation hydrogenation hydration ☐ halohydrin formation addition halogenation formation chelation hydrogenation halohydrin formation substitution hydration halogenation addition Ohalohydrin formation subtraction halogenation addition hydrogenation hydration F2 80 F3 σ F4 F5 F6 1 ! 2 # 3 $ 4 % 05 Q W & Å © 2025 McGraw Hill LLC. All Rights Reserved. F7 F8 ( 6 7 8 9 LU E R T Y U A F9arrow_forwardShow the mechanism steps to obtain the lowerenergy intermediate: *see imagearrow_forward
- Soap is made by the previous reaction *see image. The main difference between one soap and another soap isthe length (number of carbons) of the carboxylic acid. However, if a soap irritates your skin, they mostlikely used too much lye.Detergents have the same chemical structure as soaps except for the functional group. Detergentshave sulfate (R-SO4H) and phosphate (R-PO4H2) functional groups. Draw the above carboxylic acidcarbon chain but as the two variants of detergents. *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forward
- Provide the mechanism for this transformation: *see imagearrow_forwardAssign all the signals individually (please assign the red, green and blue)arrow_forwardThe two pKa values of oxalic acid are 1.25 and 3.81. Why are they not the same value? Show the protontransfer as part of your explanation. *see imagearrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning


