
Chemistry for Today: General Organic and Biochemistry
9th Edition
ISBN: 9781337514576
Author: Seager
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 17, Problem 17.6E
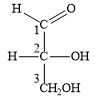
Why are carbon atoms
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
help me solve this HW
Molecules of the form AH2 can exist in two potential geometries: linear or bent. Construct molecular orbital diagrams for linear and bent CH2. Identify the relevant point group, include all of the appropriate symmetry labels and pictures, and fill in the electrons. Which geometry would you predict to be more stable, and why? (Please draw out the diagram and explain)
Indicate the variation in conductivity with concentration in solutions of strong electrolytes and weak electrolytes.
Chapter 17 Solutions
Chemistry for Today: General Organic and Biochemistry
Ch. 17 - Prob. 17.1ECh. 17 - Describe whether each of the following substances...Ch. 17 - Prob. 17.3ECh. 17 - Prob. 17.4ECh. 17 - Prob. 17.5ECh. 17 - Why are carbon atoms 1 and 3 of glyceraldehyde not...Ch. 17 - Prob. 17.7ECh. 17 - Which of the following molecules can have...Ch. 17 - Which of the following molecules can have...Ch. 17 - Prob. 17.10E
Ch. 17 - Prob. 17.11ECh. 17 - Prob. 17.12ECh. 17 - Prob. 17.13ECh. 17 - Draw Fischer projections for both the D and L...Ch. 17 - Prob. 17.15ECh. 17 - Prob. 17.16ECh. 17 - Prob. 17.17ECh. 17 - Prob. 17.18ECh. 17 - Prob. 17.19ECh. 17 - Prob. 17.20ECh. 17 - Prob. 17.21ECh. 17 - Prob. 17.22ECh. 17 - Prob. 17.23ECh. 17 - Prob. 17.24ECh. 17 - Prob. 17.25ECh. 17 - Prob. 17.26ECh. 17 - Prob. 17.27ECh. 17 - Prob. 17.28ECh. 17 - Prob. 17.29ECh. 17 - Prob. 17.30ECh. 17 - Prob. 17.31ECh. 17 - Prob. 17.32ECh. 17 - Prob. 17.33ECh. 17 - Prob. 17.34ECh. 17 - Prob. 17.35ECh. 17 - Prob. 17.36ECh. 17 - Prob. 17.37ECh. 17 - Prob. 17.38ECh. 17 - Prob. 17.39ECh. 17 - Prob. 17.40ECh. 17 - Prob. 17.41ECh. 17 - Prob. 17.42ECh. 17 - Prob. 17.43ECh. 17 - Prob. 17.44ECh. 17 - Prob. 17.45ECh. 17 - Prob. 17.46ECh. 17 - Prob. 17.47ECh. 17 - Sucrose and honey are commonly used sweeteners....Ch. 17 - Prob. 17.49ECh. 17 - Prob. 17.50ECh. 17 - Prob. 17.51ECh. 17 - Prob. 17.52ECh. 17 - Prob. 17.53ECh. 17 - Prob. 17.54ECh. 17 - Prob. 17.55ECh. 17 - Prob. 17.56ECh. 17 - Prob. 17.57ECh. 17 - Prob. 17.58ECh. 17 - Prob. 17.59ECh. 17 - Prob. 17.60ECh. 17 - Prob. 17.61ECh. 17 - Prob. 17.62ECh. 17 - Prob. 17.63ECh. 17 - Prob. 17.64ECh. 17 - Prob. 17.65ECh. 17 - Prob. 17.66ECh. 17 - Prob. 17.67ECh. 17 - Prob. 17.68ECh. 17 - Prob. 17.69ECh. 17 - Prob. 17.70ECh. 17 - Prob. 17.71ECh. 17 - Prob. 17.72ECh. 17 - Prob. 17.73ECh. 17 - Glucose is a reducing sugar, which if boiled in...Ch. 17 - Prob. 17.75E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The molar conductivity of a very dilute solution of NaCl has been determined. If it is diluted to one-fourth of the initial concentration, qualitatively explain how the molar conductivity of the new solution will compare with the first.arrow_forwardWhat does the phrase mean, if instead of 1 Faraday of electricity, Q coulombs (Q/F Faradays) pass through?arrow_forwardWhat characteristics should an interface that forms an electrode have?arrow_forward
- For a weak acid AcH, calculate the dissociated fraction (alpha), if its concentration is 1.540 mol L-1 and the concentration [H+] is 5.01x10-4 mol L-1.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forward
- If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardDetermine the distance between the metal and the OHP layer using the Helm- holtz model when the electrode's differential capacitance is 145 μF cm². DATA: dielectric constant of the medium for the interfacial zone &r= lectric constant of the vacuum &0 = 8.85-10-12 F m-1 = 50, die-arrow_forwardDescribe a sequence of photophysical processes that can be followed by radiation adsorbed by a molecule in the ground state to give rise to phosphorescent emission.arrow_forward
- State two similarities between fluorescence and phosphorescence.arrow_forwardState three photophysical processes that can be related to the effects of incident radiation on a molecule in its ground state. Consider that radiation can give rise to fluorescent emission, but not phosphorescent emission.arrow_forwardIn a photochemical reaction, how is the rate of the process related to its quantum yield?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY