
Concept explainers
17-54 Following is the structure of immunosuppressant FK-506, a molecule shown to disrupt calcineurin-mediated signal transduction in T-lymphocytes.
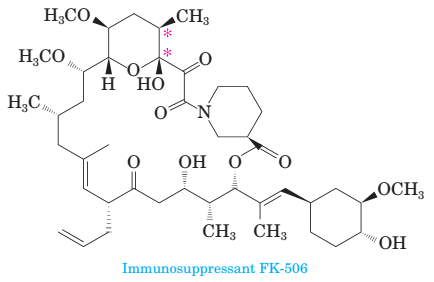
(a) There are three carbon—carbon double bonds present in this molecule. Which of the three has the potential for cis/trans isomerism? Assign a cis or trans con?guration to each carbon-carbon double bond that has this possibility.
(b) How many stereocenters are present in this molecule? How many stereoisomers are possible for it?
(c) Are there any aromatic components in this molecule?
(d) Consider the two carbon atoms marked with asterisks. Assign an R or S con?guration of each stereocenter.
(e) Because of the presence of a 21-member ring, this molecule is described as a macrocycle. This ring is fashioned by three types of bonds, several carbon-carbon bonds, one ester, one hemiacetal, and one amide. Locate the ester and the hemiacetal.
(f) Draw the structural formula of the long chain compound that would result if the hemiacetal were to be cleaved to an alcohol and a carbonyl group.
Trending nowThis is a popular solution!

Chapter 16 Solutions
INTRO.TO GENERAL,ORGAN...-OWLV2 ACCESS
- A pdf file of your hand drawn, stepwise mechanisms for the reactions. For each reaction in the assignment, you must write each mechanism three times (there are 10 reactions, so 30 mechanisms). (A) do the work on a tablet and save as a pdf., it is expected to write each mechanism out and NOT copy and paste the mechanism after writing it just once. Everything should be drawn out stepwise and every bond that is formed and broken in the process of the reaction, and is expected to see all relevant lone pair electrons and curved arrows. Aldol: NaOH HO H Δ NaOH Δarrow_forwardNonearrow_forwardDraw structures corresponding to the following names and give IUPAC names for the following compounds: (8 Point) a) b) c) CH3 CH2CH3 CH3CHCH2CH2CH CH3 C=C H3C H H2C=C=CHCH3 d) CI e) (3E,5Z)-2,6-Dimethyl-1,3,5,7-octatetraene f) (Z)-4-bromo-3-methyl-3-penten-1-yne g) cis-1-Bromo-2-ethylcyclopentane h) (5R)-4,4,5-trichloro-3,3-dimethyldecanearrow_forward
- Draw a Newman projection from carbon 3 to carbon 2 in the highest energy conformation for the following molecule. What is this conformation called? What kind of strain is present? Brarrow_forwardWhich of the following dienophiles is most reactive in a Diels-Alder reaction: Please explain why the correct answer to this question is option 5. Please provide a detailed explanation.arrow_forwardWhich of the following would you expect to be aromatic? Please provide a detailed explanation.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



