
(a)
Interpretation:
Write a structural formula for the principal organic product formed by treating the following compound with
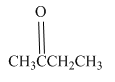
Concept Introduction:
Reduction is the process in which hydrogen is added to the compound.
(b)
Interpretation:
Write a structural formula for the principal organic product formed by treating with following compound with
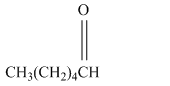
Concept Introduction:
Reduction is the process in which hydrogen is added to the compound.
(c)
Interpretation:
Write a structural formula for the principal organic product formed by treating with following compound with
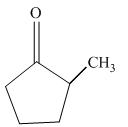
Concept Introduction:
Reduction is the process in which hydrogen is added to the compound.
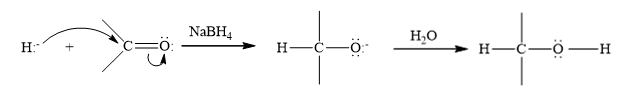
(d)
Interpretation:
Write a structural formula for the principal organic product formed by treating with following compound with
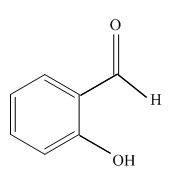
Concept Introduction:
Reduction is the process in which hydrogen is added to the compound.
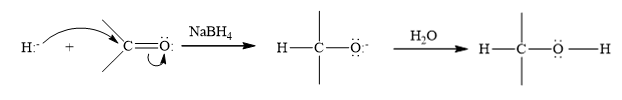
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
INTRO.TO GENERAL,ORGAN...-OWLV2 ACCESS
- X Draw the major products of the elimination reaction below. If elimination would not occur at a significant rate, check the box under the drawing area instead. ది www. Cl + OH Elimination will not occur at a significant rate. Click and drag to start drawing a structure.arrow_forwardNonearrow_forward1A H 2A Li Be Use the References to access important values if needed for this question. 8A 3A 4A 5A 6A 7A He B C N O F Ne Na Mg 3B 4B 5B 6B 7B 8B-1B 2B Al Si P 1B 2B Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe * Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Rf Ha ****** Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Analyze the following reaction by looking at the electron configurations given below each box. Put a number and a symbol in each box to show the number and kind of the corresponding atom or ion. Use the smallest integers possible. cation anion + + Shell 1: 2 Shell 2: 8 Shell 3: 1 Shell 1 : 2 Shell 2 : 6 Shell 1 : 2 Shell 2: 8 Shell 1: 2 Shell 2: 8arrow_forward
- Nonearrow_forwardIV. Show the detailed synthesis strategy for the following compounds. a. CH3CH2CH2CH2Br CH3CH2CCH2CH2CH3arrow_forwardDo the electrons on the OH participate in resonance with the ring through a p orbital? How many pi electrons are in the ring, 4 (from the two double bonds) or 6 (including the electrons on the O)?arrow_forward
- Predict and draw the product of the following organic reaction:arrow_forwardNonearrow_forwardRedraw the molecule below as a skeletal ("line") structure. Be sure to use wedge and dash bonds if necessary to accurately represent the direction of the bonds to ring substituents. Cl. Br Click and drag to start drawing a structure. : ☐ ☑ Parrow_forward
- K m Choose the best reagents to complete the following reaction. L ZI 0 Problem 4 of 11 A 1. NaOH 2. CH3CH2CH2NH2 1. HCI B OH 2. CH3CH2CH2NH2 DII F1 F2 F3 F4 F5 A F6 C CH3CH2CH2NH2 1. SOCl2 D 2. CH3CH2CH2NH2 1. CH3CH2CH2NH2 E 2. SOCl2 Done PrtScn Home End FA FQ 510 * PgUp M Submit PgDn F11arrow_forwardNonearrow_forwardPlease provide a mechanism of synthesis 1,4-diaminobenzene, start from a benzene ring.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

