
Concept explainers
a)
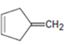
Interpretation:
Whether 4-methylenecyclopent-1-ene will have a π – π * UV adsorption in the 200 to 400nm range is to be stated.
Concept introduction:
Compounds having alternating double bonds (conjugated dienes) will have π – π *UV adsorption in the 200 to 400nm range.
To state:
Whether 4-methylenecyclopent-1-ene will have a π – π * UV adsorption in the 200 to 400nm range.
b)
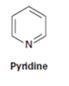
Interpretation:
Whether pyridine will have a π – π * UV adsorption in the 200 to 400nm range
Concept introduction:
Compounds having alternating double bonds (conjugated dienes) will have π – π * UV adsorption in the 200 to 400nm range.
To state:
Whether pyridine will have a π – π * UV adsorption in the 200 to 400nm range
c)
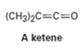
Interpretation:
Whether the ketene given will have a π – π * UV adsorption in the 200 to 400 nm range
Concept introduction:
Compounds having alternating double bonds (conjugated dienes) will have π – π * UV adsorption in the 200 to 400nm range.
To state:
Whether the ketene given will have a π – π * UV adsorption in the 200 to 400 nm range
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Organic Chemistry
- Provide the drawing of the unknown structure that corresponds with this data.arrow_forward20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forwardGiven the attached data, provide the drawing for the corresponding structure.arrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


