
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 14.SE, Problem 61AP
Interpretation Introduction
Interpretation:
The concentration of the ergosterol solution whose absorbance A= 0.065 with a sample pathlength l = 1.00 cm is to be calculated if ergosterol has a UV absorption maxima of 282 nm and mole absorptivity ε = 11,900.
Concept introduction:
The molar absorptivity, ε, is given by 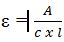 where A is absorbance, C is the concentration of the solution in moles/lit and l is the sample path length in cm.
where A is absorbance, C is the concentration of the solution in moles/lit and l is the sample path length in cm.
To calculate:
The concentration of the ergosterol solution whose absorbance A= 0.065 with a sample pathlength l = 1.00 cm, if ergosterol has a UV absorption maxima of 282 nm and mole absorptivity ε = 11,900.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
1. Determine the relationship between the following molecules as identical, diastereomers, or enantiomers (6
points, 2 points each).
OH
OH
OH
A-A
OH
HOT
HO-
ACHN
and
HO-
ACHN
OH
HO
HO
°
OH
and
OH
OH
SH
and
...SH
20,0
Complete the electron pushing mechanism to
y drawing the necomery unicaciones and carved on for
Step 1: Add curved arms for the tint step, traiment with NalilĻ. The Nation
458
Step 2: Added for the second step, inalment with), how the "counterion
bar
Step 3: Daw the products of the last simplom organic and one incoganic spacient, including all nonbonding
please provide the structure for this problem, thank you!
Chapter 14 Solutions
Organic Chemistry
Ch. 14.1 - Prob. 1PCh. 14.2 - Give the structures of both 1, 2 and 1, 4 adducts...Ch. 14.2 - Prob. 3PCh. 14.2 - Give the structures of both 1, 2 and 1, 4 adducts...Ch. 14.3 - Prob. 5PCh. 14.3 - Prob. 6PCh. 14.5 - Predict the product of the following Diels–Alder...Ch. 14.5 - Prob. 8PCh. 14.5 - Which of the following dienes have an s-cis...Ch. 14.5 - Predict the product of the following Diels–Alder...
Ch. 14.6 - Prob. 11PCh. 14.6 - Prob. 12PCh. 14.7 - Prob. 13PCh. 14.7 - Prob. 14PCh. 14.8 - Which of the following compounds would you expect...Ch. 14.SE - Prob. 16VCCh. 14.SE - Show the product of the Diels–Alder reaction of...Ch. 14.SE - Prob. 18VCCh. 14.SE - Prob. 19VCCh. 14.SE - Prob. 20MPCh. 14.SE - Prob. 21MPCh. 14.SE - In light of your answer to Problem 14-21 propose...Ch. 14.SE - Luminol, which is used by forensic scientists to...Ch. 14.SE - Prob. 24MPCh. 14.SE - Give IUPAC names for the following compounds:Ch. 14.SE - Prob. 26APCh. 14.SE - Prob. 27APCh. 14.SE - Electrophilic addition of Br2 to isoprene...Ch. 14.SE - Prob. 29APCh. 14.SE - Prob. 30APCh. 14.SE - Predict the products of the following...Ch. 14.SE - 2,3-Di-tert-butyl-1,3-butadiene does not undergo...Ch. 14.SE - Prob. 33APCh. 14.SE - Prob. 34APCh. 14.SE - Prob. 35APCh. 14.SE - Prob. 36APCh. 14.SE - Rank the following dienophiles in order of their...Ch. 14.SE - Prob. 38APCh. 14.SE - Prob. 39APCh. 14.SE - Prob. 40APCh. 14.SE - Although the Diels–Alder reaction generally...Ch. 14.SE - Prob. 42APCh. 14.SE - Tires whose sidewalls are made of natural rubber...Ch. 14.SE - Prob. 44APCh. 14.SE - Prob. 45APCh. 14.SE - Prob. 46APCh. 14.SE - Would you expect allene, H2C = C = CH2, to show a...Ch. 14.SE - The following ultraviolet absorption maxima have...Ch. 14.SE - Prob. 49APCh. 14.SE - -Ocimene is a pleasant-smelling hydrocarbon found...Ch. 14.SE - Draw the resonance forms that result when the...Ch. 14.SE - Prob. 52APCh. 14.SE - Treatment of 3,4-dibromohexane with strong base...Ch. 14.SE - Prob. 54APCh. 14.SE - Prob. 55APCh. 14.SE - Prob. 56APCh. 14.SE - Prob. 57APCh. 14.SE - Prob. 58APCh. 14.SE - Hydrocarbon A, C10H14, has a UV absorption at...Ch. 14.SE - Prob. 60APCh. 14.SE - Prob. 61APCh. 14.SE - Prob. 62APCh. 14.SE - Prob. 63APCh. 14.SE - Prob. 64APCh. 14.SE - The double bond of an enamine (alkene + amine) is...Ch. 14.SE - Prob. 66AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the Fischer projection from the skeletal structure shown below. HO OH OH OH OH H Q Drawing Atoms, Bonds and Rings Charges I ☐ T HO H H OH HO I CH2OH H OH Drag H OH -CH2OH CHO -COOH Undo Reset Remove Donearrow_forwardplease provide the structure for this problem, thank youarrow_forwardpresented by Morallen Lig Intermine the hand product for the given mution by adding atoms, bonds, nonhonding diarion panda скуль Step 3: Comp the draw the product Step 2: Agama workup Compithe 429 ملولةarrow_forward
- Reaction A 0,0arrow_forwardpresented by Morillon Leaning Predict the organic product for the min кусур HSC Adithane carved arnown to come than that to the condon slchroruis in acid in in aquishri with ноюarrow_forward6.15PM Sun Mar 30 K Draw the major product of this reaction. Include any relevant stereochemistry. Ignore inorganic byproducts. Problem 1 of O H [PhзPCH2CH3]*C|¯ NaH Drawing > Q Atoms, Bonds and Draw or tap a nearrow_forward
- 8:17 PM Sun Mar 30 Draw the major product of this reaction. Ignore inorganic byproducts. HSCH2CH2CH2SH, BF3 Probler Drawing Ato Bonds Clarrow_forwardpresented by Mr L How the coprion. (Il Done in no wraction, dew the starting redential) доarrow_forward8:16 PM Sun Mar 30 K Draw the major product of this reaction. Ignore inorganic byproducts. Proble 1. CH3MgBr 2. H3O+ F Drawingarrow_forward
- о но оarrow_forwardName the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forwardMacmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
