
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14.SE, Problem 51AP
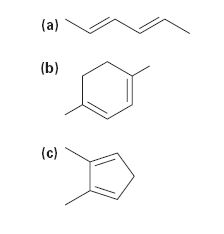
Draw the resonance forms that result when the dienes below are protonated. If the resonance forms differ in energy, identify the most stable one.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
If we assume a system with an anodic overpotential, the variation of n as a function
of current density:
1. at low fields is linear 2. at higher fields, it follows Tafel's law
Obtain the range of current densities for which the overpotential has the same value
when calculated for 1 and 2 cases (maximum relative difference of 5% compared to
the behavior for higher fields).
To which overpotential range does this correspond?
Data: i = 1.5 mA cm², T = 300°C, B = 0.64, R = 8.314 J K1 mol-1 and F = 96485 C mol-1.
Answer by equation please
Some of the theories used to describe interface structure can be distinguished by:1. the measured potential difference.2. the distribution of ions in solution.3. the calculation of charge density.4. the external Helmoltz plane.
Chapter 14 Solutions
Organic Chemistry
Ch. 14.1 - Prob. 1PCh. 14.2 - Give the structures of both 1, 2 and 1, 4 adducts...Ch. 14.2 - Prob. 3PCh. 14.2 - Give the structures of both 1, 2 and 1, 4 adducts...Ch. 14.3 - Prob. 5PCh. 14.3 - Prob. 6PCh. 14.5 - Predict the product of the following Diels–Alder...Ch. 14.5 - Prob. 8PCh. 14.5 - Which of the following dienes have an s-cis...Ch. 14.5 - Predict the product of the following Diels–Alder...
Ch. 14.6 - Prob. 11PCh. 14.6 - Prob. 12PCh. 14.7 - Prob. 13PCh. 14.7 - Prob. 14PCh. 14.8 - Which of the following compounds would you expect...Ch. 14.SE - Prob. 16VCCh. 14.SE - Show the product of the Diels–Alder reaction of...Ch. 14.SE - Prob. 18VCCh. 14.SE - Prob. 19VCCh. 14.SE - Prob. 20MPCh. 14.SE - Prob. 21MPCh. 14.SE - In light of your answer to Problem 14-21 propose...Ch. 14.SE - Luminol, which is used by forensic scientists to...Ch. 14.SE - Prob. 24MPCh. 14.SE - Give IUPAC names for the following compounds:Ch. 14.SE - Prob. 26APCh. 14.SE - Prob. 27APCh. 14.SE - Electrophilic addition of Br2 to isoprene...Ch. 14.SE - Prob. 29APCh. 14.SE - Prob. 30APCh. 14.SE - Predict the products of the following...Ch. 14.SE - 2,3-Di-tert-butyl-1,3-butadiene does not undergo...Ch. 14.SE - Prob. 33APCh. 14.SE - Prob. 34APCh. 14.SE - Prob. 35APCh. 14.SE - Prob. 36APCh. 14.SE - Rank the following dienophiles in order of their...Ch. 14.SE - Prob. 38APCh. 14.SE - Prob. 39APCh. 14.SE - Prob. 40APCh. 14.SE - Although the Diels–Alder reaction generally...Ch. 14.SE - Prob. 42APCh. 14.SE - Tires whose sidewalls are made of natural rubber...Ch. 14.SE - Prob. 44APCh. 14.SE - Prob. 45APCh. 14.SE - Prob. 46APCh. 14.SE - Would you expect allene, H2C = C = CH2, to show a...Ch. 14.SE - The following ultraviolet absorption maxima have...Ch. 14.SE - Prob. 49APCh. 14.SE - -Ocimene is a pleasant-smelling hydrocarbon found...Ch. 14.SE - Draw the resonance forms that result when the...Ch. 14.SE - Prob. 52APCh. 14.SE - Treatment of 3,4-dibromohexane with strong base...Ch. 14.SE - Prob. 54APCh. 14.SE - Prob. 55APCh. 14.SE - Prob. 56APCh. 14.SE - Prob. 57APCh. 14.SE - Prob. 58APCh. 14.SE - Hydrocarbon A, C10H14, has a UV absorption at...Ch. 14.SE - Prob. 60APCh. 14.SE - Prob. 61APCh. 14.SE - Prob. 62APCh. 14.SE - Prob. 63APCh. 14.SE - Prob. 64APCh. 14.SE - The double bond of an enamine (alkene + amine) is...Ch. 14.SE - Prob. 66AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?arrow_forwardUsing the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$arrow_forwardIndicate characteristics of oxodec acid.arrow_forward
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY