
Concept explainers
(a)
Interpretation:
The
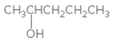
Concept Introduction:
When H2O is lost from a material it is called dehydration. When an alcohol is treated with a strong acid such as H2SO4, a water molecule is lost by breaking two adjacent bonds C-OH and C-H and forming a new double bond, producing an alkene. This is an elimination reaction.
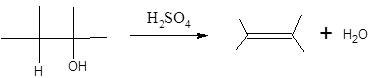
In this reaction more than one type of alkene may produce. But one of them is the major product.
According to Zaitsev rule, the major product alkene formed by elimination, is that which possess more alkyl groups bonded to it
(b)
Interpretation:
The alkene formed when the given alcohol is treated with H2SO4 should be determined. The major product should be predicted using Zaitsev rule.
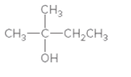
Concept Introduction:
When H2O is lost from a material it is called dehydration. When an alcohol is treated with a strong acid such as H2SO4, a water molecule is lost by breaking two adjacent bonds C-OH and C-H and forming a new double bond, producing an alkene. This is an elimination reaction.
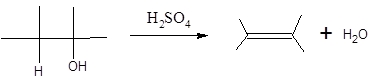
In this reaction more than one type of alkene may produce. But one of them is the major product.
According to Zaitsev rule, the major product alkene formed by elimination, is that which possess more alkyl groups bonded to it
(c)
Interpretation:
The alkene formed when the given alcohol is treated with H2SO4 should be determined. The major product should be predicted using Zaitsev rule.
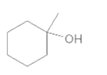
Concept Introduction:
When H2O is lost from a material it is called dehydration. When an alcohol is treated with a strong acid such as H2SO4, a water molecule is lost by breaking two adjacent bonds C-OH and C-H and forming a new double bond, producing an alkene. This is an elimination reaction.
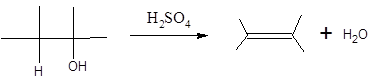
In this reaction more than one type of alkene may produce. But one of them is the major product.
According to Zaitsev rule, the major product alkene formed by elimination, is that which possess more alkyl groups bonded to it
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
General, Organic, & Biological Chemistry
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
